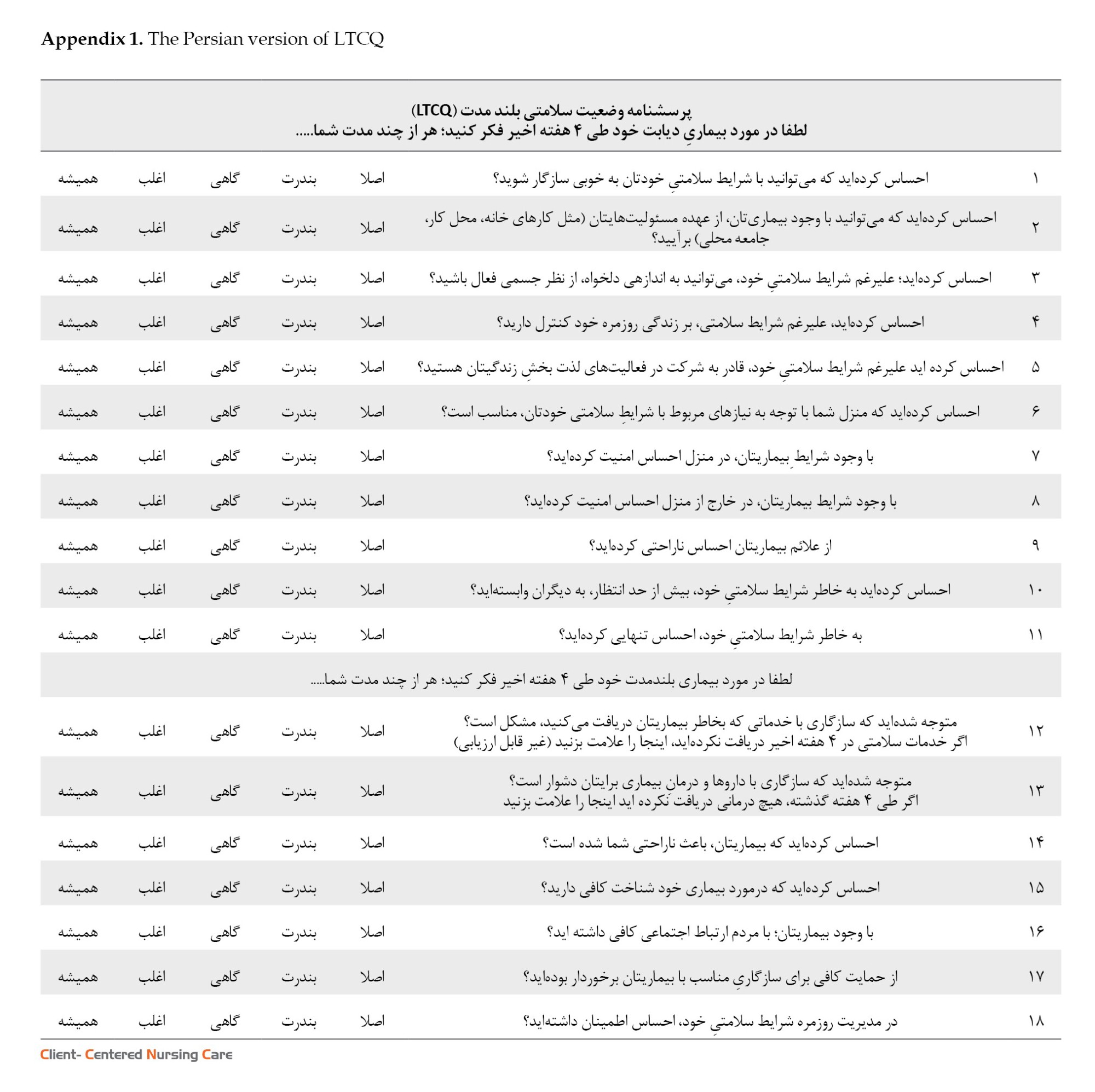
Mon, Dec 8, 2025
[Archive]
Volume 11, Issue 2 (Spring 2025)
JCCNC 2025, 11(2): 113-124 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Iloonkashkooli R, Hadian Shirazi Z, Soltanian M, Setoodeh G, Momennasab M. Cultural Adaptation and Psychometric Properties of Long-term Conditions Questionnaire for Patients With Diabetes Mellitus. JCCNC 2025; 11 (2) :113-124
URL: http://jccnc.iums.ac.ir/article-1-590-en.html
URL: http://jccnc.iums.ac.ir/article-1-590-en.html
Raziyeh Iloonkashkooli *1 

 , Zahra Hadian Shirazi2
, Zahra Hadian Shirazi2 

 , Mitra Soltanian3
, Mitra Soltanian3 

 , Giti Setoodeh4
, Giti Setoodeh4 
 , Marziyeh Momennasab3
, Marziyeh Momennasab3 




 , Zahra Hadian Shirazi2
, Zahra Hadian Shirazi2 

 , Mitra Soltanian3
, Mitra Soltanian3 

 , Giti Setoodeh4
, Giti Setoodeh4 
 , Marziyeh Momennasab3
, Marziyeh Momennasab3 


1- Student Research Committee, School of Nursing and Midwifery, Shiraz University of Medical Sciences, Shiraz, Iran. , iloonraziye@gmail.com
2- Department of Nursing, Committee-Based Psychiatric Care Research Center, School of Nursing and Midwifery, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Department of Nursing, School of Nursing and Midwifery, Shiraz University of Medical Sciences, Shiraz, Iran.
4- Department of Mental Health and Psychiatric Nursing, School of Nursing and Midwifery, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Department of Nursing, Committee-Based Psychiatric Care Research Center, School of Nursing and Midwifery, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Department of Nursing, School of Nursing and Midwifery, Shiraz University of Medical Sciences, Shiraz, Iran.
4- Department of Mental Health and Psychiatric Nursing, School of Nursing and Midwifery, Shiraz University of Medical Sciences, Shiraz, Iran.
Keywords: Diabetes mellitus (DM), Validation study, Psychometric evaluation, Reliability, Cultural adaptation
Full-Text [PDF 806 kb]
(345 Downloads)
| Abstract (HTML) (1436 Views)
Full-Text: (254 Views)
Introduction
Diabetes mellitus has become a significant global public health issue. According to the International Diabetes Federation (IDF), 536.6 million people were living with diabetes in 2021, and this figure is expected to rise by 46%, reaching 783.2 million by 2045 (Zan et al., 2024). Approximately 4.2 million deaths among adults aged 20 to 79 are due to diabetes. Globally, diabetes is responsible for an estimated 11.3% of deaths, with nearly half (46.2%) of these occurring in individuals under 60 (Saeedi et al., 2020). Due to its chronic nature, diabetes requires long-term care, continuous monitoring of self-management and support to prevent acute complications and minimize the risk of long-term complications. Health specialists recognize that managing diabetes is intricate and involves addressing numerous factors beyond merely controlling blood sugar levels (Powers et al., 2020).
Not only in Iran, but also in many countries around the world, including Asian countries, the lack of identifying diabetic patients affects the individual’s life, leading to inadequate follow-up and treatment, lacking necessary coordination in disease management, and ultimately significantly increasing diabetes-related mortality (Larijani et al., 2017). Understanding the impact of long-term conditions (LTCs) on the lives of patients with diabetes mellitus (DM) demonstrates the need for multifaceted health interventions and necessitates policymaking to improve treatment by health care providers (Hill-Briggs et al., 2021; Papaspurou et al., 2015). Any health care intervention that can delay the onset of diabetes symptoms or slow the progression of its complications will play a significant role in alleviating patients’ suffering, improving their quality of life (QOL), and reducing the associated costs (Daneshkohan et al., 2019). In this regard, understanding the long-term health conditions of patients with DM is an initial assessment that determines the impact of diabetes on patients’ lives over time and how it deviates them from living in optimal health conditions. Using this knowledge, health care providers will be able to establish long-term care plans for these patients based on the impact of diabetes on their lives, leading to the prevention of complications as much as possible (Ambrosio et al., 2023; Tamornpark et al., 2022).
The LTCs questionnaire (LTCQ) was developed as a patient-reported outcome measure to understand the experience of a satisfactory life with one or more long-term health conditions and multimorbidity (Potter et al., 2017). The questionnaire includes 20 questions that assess the effects of living with one or more long-term mental or physical conditions. It was designed for use in both health and social care contexts. Answers are scored on a scale from 0 (most negative) to 4 (most positive). The scores of all 20 questions are combined into a single overall score, with higher scores reflecting more positive life experiences (Kelly et al., 2022). It is a concise, self-reporting tool to measure the overall impact of long-term conditions (Garcimartin et al., 2017). It was designed to cover both traditional and non-traditional domains that enhance health-related QOL, within a broad concept of “maintaining well-being with LTCs.” The questionnaire complements symptom burden assessments through disease-specific measures. The development of LTCQ involved a series of steps, including literature reviews, stakeholder and public consultation, qualitative interviews with patients, a translatability evaluation, and an initial validation survey (Potter et al., 2021).
The availability of a native questionnaire to evaluate the LTCs of diabetic patients helps health policymakers and health care providers in Iran make informed decisions for health policy and long-term care planning based on the insights gained from these evaluations. Since no study has been conducted in Iran to validate the Persian version of LTCQ, and chronic diseases including diabetes are on the rise, this study aims to perform the cultural adaptation and assess the psychometric properties of the LTCQ in patients with DM.
Materials and Methods
Study design
A methodological study was carried out to evaluate the psychometric properties of LTCQ. It is an instrument to assess ‘living well’ in the context of chronic illness. As a generic patient-reported outcome measure contributing to a wide range of areas across both health care and social services, the LTCQ can meet the distinct need for comprehensive outcome measurement that makes integrated service provision possible. This tool was developed in 2016 by potter and colleagues. The validity and reliability of the questionnaire have been confirmed, with a Cronbach α value of 0.95 and a confidence interval (CI) of 93% to 95%. The questionnaire comprises 20 items, each rated on a 5-point Likert scale ranging from “never=0” to “always=4” with higher scores indicating more favorable living conditions with the disease. The tool’s scores are adjusted to produce a total LTCQ score between 0 and 100 (Potter et al., 2017).
The current study was carried out in two phases. Initially, a forward-backward translation method was applied (Bradley, 2013; WHO, 2023), and the original version of LTCQ was translated into Persian (see Appendix 1). In the second phase, the psychometric properties of the translated instrument were measured.
A cross-sectional study was designed to evaluate the construct validity and reliability of the questionnaire. The subjects were 205 patients with type 2 diabetes selected from the diabetes clinic of Shahid Beheshti Hospital, Shiraz City, Iran, by simple random sampling. The sample size was determined to meet the suggested minimum number of items per case (5-10 items) to ensure the validity of the factor analysis process (Munro, 2005; Yong & Pearce, 2013). The study included individuals with an active profile in the diabetes clinic, aged over 30, and of native Iranian nationality. Participants with major mental disorders, such as Alzheimer, congenital mental retardation, or severe disabilities, like quadriplegia or serious cardiovascular conditions, were excluded. The research team confirmed these criteria to ensure accurate sampling.
Forward-backward translation and cross-cultural adaptation
The instrument was translated from English into Persian following the World Health Organization (WHO) forward-backward translation standard protocol, with prior permission obtained from the corresponding author, as one of the instrument designers (the process of translation and adaptation of instruments, 2009). two fluent English experts in medical sciences were recruited to translate the questionnaire into Persian. After making the necessary corrections in the two translated versions, two other English-speaking experts unaware of the original English text, were requested to translate the Persian version back into English. The resulting translations were compared to each other and to the original text to ensure accuracy in translation. The research team made minor wording and terminology changes in coordination with the translation and re-translation teams. A total of 10 type 2 diabetes patients were interviewed to identify any difficulties they had in reading and understanding the questionnaire. Finally, 10 faculty members verified the cultural relevance of the questionnaire. After preparation of the initial draft of the Persian LTCQ, it was reviewed to ensure its conceptual compatibility with the original version and that the items are fit with the cultural values of Iranian society. Then, the psychometric steps of the questionnaire began.
Qualitative content validity
Content validity is often measured by relying on the knowledge of relevant experts (Zamanzadeh et al., 2014). So, the questionnaire was sent to 10 experts with knowledge and experience in instrument development, adult nursing, psychiatric nursing, and psychology to evaluate the qualitative content validity as described in the translation and cross-cultural adaptation subsection. They were asked to give their opinions about grammar, wording, item allocation, and scaling. After discussion and review by the members, the corrective comments received from the experts were reviewed and implemented.
Quantitative content validity
To evaluate the quantitative content validity of the scale, content validity ratio (CVR) and content validity index (CVI) were determined. In accordance with the Lawshe method, a 3-point scale of ‘necessary,’ ‘useful but not necessary,’ and ‘not necessary’ were utilized to determine CVR, and the items that had a score of at least 0.6 were retained in the questionnaire. To calculate the CVI, the relevance, clarity, and simplicity of each item were assessed on a 4-point Likert scale, and the corresponding index was obtained by dividing the number of experts who chose options 3 and 4 by the total number of experts. Items were accepted based on a CVI score higher than 0.8 and a CVR higher than 0.6 (Polit & Beck, 2006).
Construct validity
In the psychometric evaluation of original questionnaires by its designers, exploratory factor analysis (EFA) is performed to summarize the data, categorize them, and identify its underlying dimensions (Ahmadi et al., 2022; Brown, 2015). As the first step of construct validity assessment, the kurtosis test was performed and data normality was determined among 205 participants (Garson, 2012). Thereafter, the LTCQ factor structure was determined using EFA in SPSS software, version 23. The Kaiser–Meyer–Olkin (KMO) test and Bartlett test for sphericity were used to determine sampling adequacy and appropriateness of the factor analysis. Then, varimax rotation and maximum likelihood model were utilized to identify latent factors and select suitable items. Two items were removed from the factors due to communalities being below 0.3 (Samuels, 2017), and an 18-item questionnaire was resulted. To confirm the result of the EFA and evaluation of the 18-item scale we conducted a CFA, by utilizing structural equation modelling (SEM) in AMOS software, version 24. Therefore, the first- and second-order models were designed and fit indices were reported based on cutoff values (Bollen & Long, 1993).
Reliability
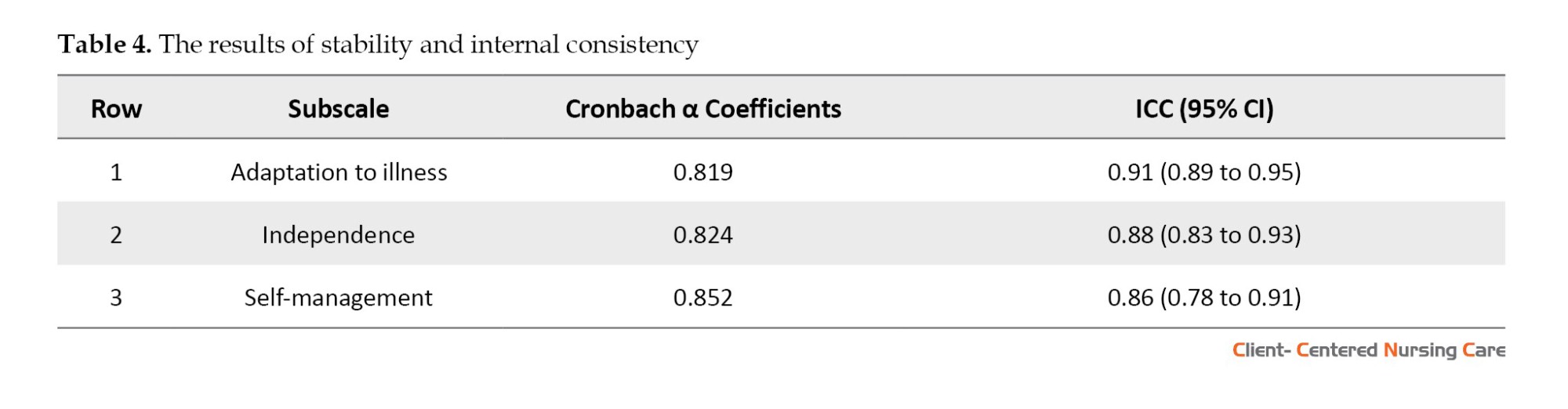
To assess the internal consistency of the questionnaire, the Cronbach α coefficient was calculated. A Cronbach α coefficient range of 0.70 to 0.95 is acceptable (Bland & Altman, 1997). The test re-test reliability assessed the stability of the measurement over time. In this study, the intraclass correlation coefficient (ICC: 0.78 - 0.95) was utilized to evaluate test re-test reliability. An ICC score above 0.80 was deemed acceptable (Koo & Li, 2016; Anselmi et al., 2019).
Results
The Persian version of the tool was developed through a forward-backward translation procedure conducted by four experts fluent in English. Ten patients with diabetes were also interviewed to ensure understanding of the content of the tool. Ultimately, the Persian version of the tool was developed for the evaluation of its psychometric properties.
Content and face validity
In the qualitative section, some health-related terms not commonly used in Iran were substituted with equivalent terms more widely recognized and accepted in our country. For example, the expression “despite health conditions” changed to “despite the disease” in some instances. This revision was done to ensure that the study materials were accessible and easily understood by all participants, regardless of their level of familiarity with medical terminology.
As mentioned before, evaluation of content validity involved a thorough consultation process with experts in the qualitative section. The experts’ suggestions were taken into account to enhance the questionnaire’s content validity, resulting in the modification of the instrument. Regarding quantitative content validity, all items in the questionnaire were analyzed, and the CVR scores for all items surpassed 0.6, while the CVI scores were greater than 0.8. The results indicated that the questionnaire had a high level of content validity and none of the items needed to be removed, demonstrating the suitability of the questionnaire.
Construct validity
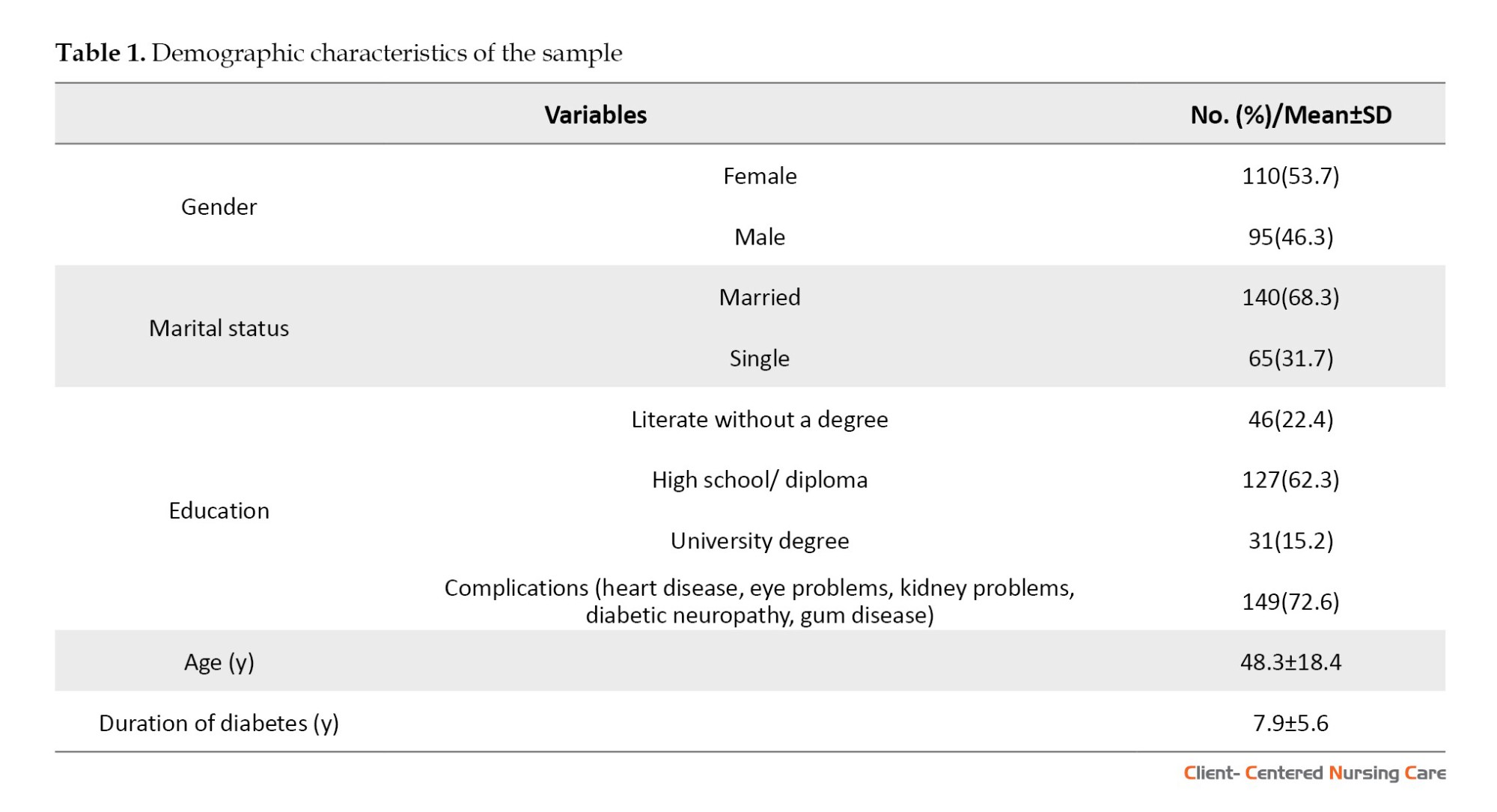
To examine construct validity, we carried out a cross-sectional study, involving a sample of 205 patients. Of these patients, over half were female (53.7%). The age of the patients ranged from 20 to 68 years, with a Mean±SD age of 48.3±18.4 years. Moreover, 84.7% of participants were individuals without a university degree, and 15.3% had some university education. The mean duration of diabetes among the participants was 7.9±5.6 years, ranging from 2 to 14 years. It was noted that the majority of the participants (72.6%) had at least one complication associated with diabetes. These demographic characteristics provided valuable insights into the study sample and facilitated the interpretation of study results (Table 1).
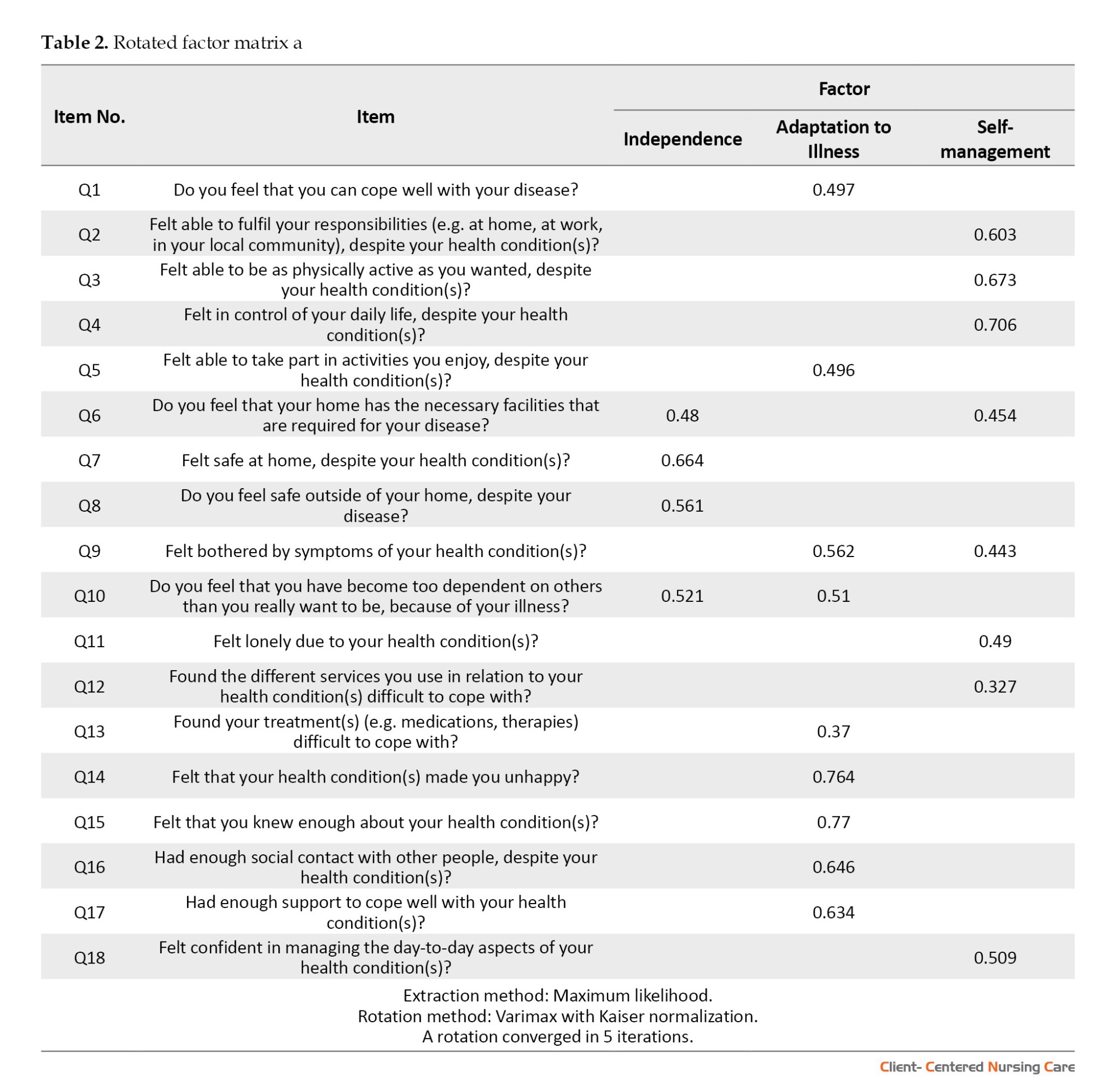
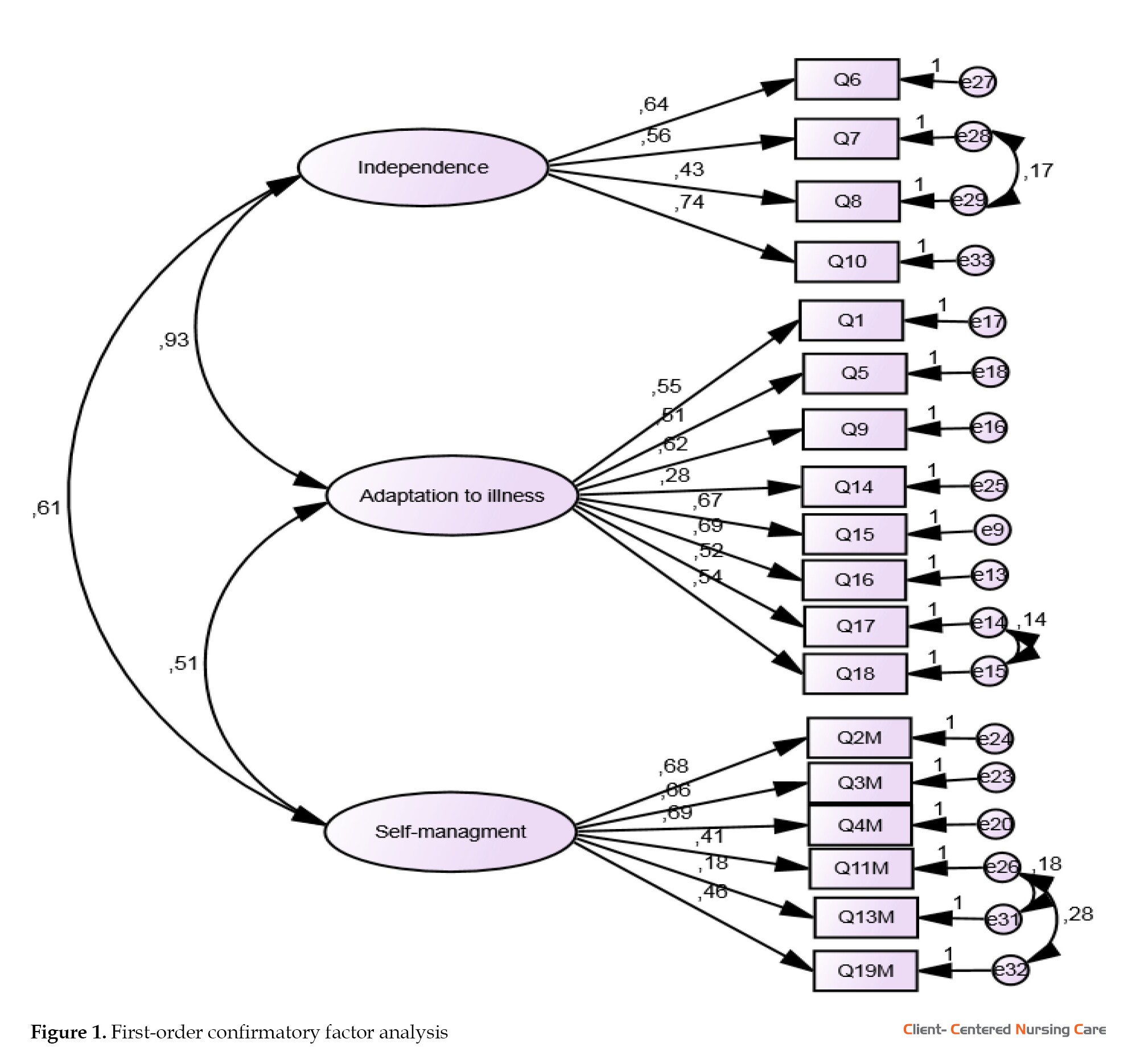
Before performing factor analysis, the initial 20-item questionnaire demonstrated a Cronbach α value of 0.852, indicating a high level of internal consistency. Using the kurtosis test, it was determined that all the items fell within the acceptable range of -1 to 1, and therefore, no items were removed. Furthermore, the normality test and boxplot analysis identified 3 participants (45, 48, and 197) as consistent outliers across multiple items. These participants were subsequently excluded from the dataset. Regarding the results of EFA, good results of Bartlett test of sphericity (χ2=1386.523; P<0.001) and the KMO (0.85) showed sampling adequacy and provided minimum standards for conducting a factor analysis (Bucci et al., 2018; Field, 2013). Having excluded 3 participants, we proceeded with EFA, anticipating a three-factor structure. We assessed item suitability under these factors, eliminating items with loadings below 0.30. After utilizing varimax rotation, the maximum likelihood model demonstrated superior statistical and theoretical adequacy (Wood et al., 1996). In particular, items 12 and 20 were omitted from the analysis due to being inadequately fit. EFA extracted 3 factors. Using a panel of professionals in health promotion and psychology, we named these 3 factors. They were categorized as independence, adaptation to illness, and self-management. Main factors of LTCQ are presented in Table 2.
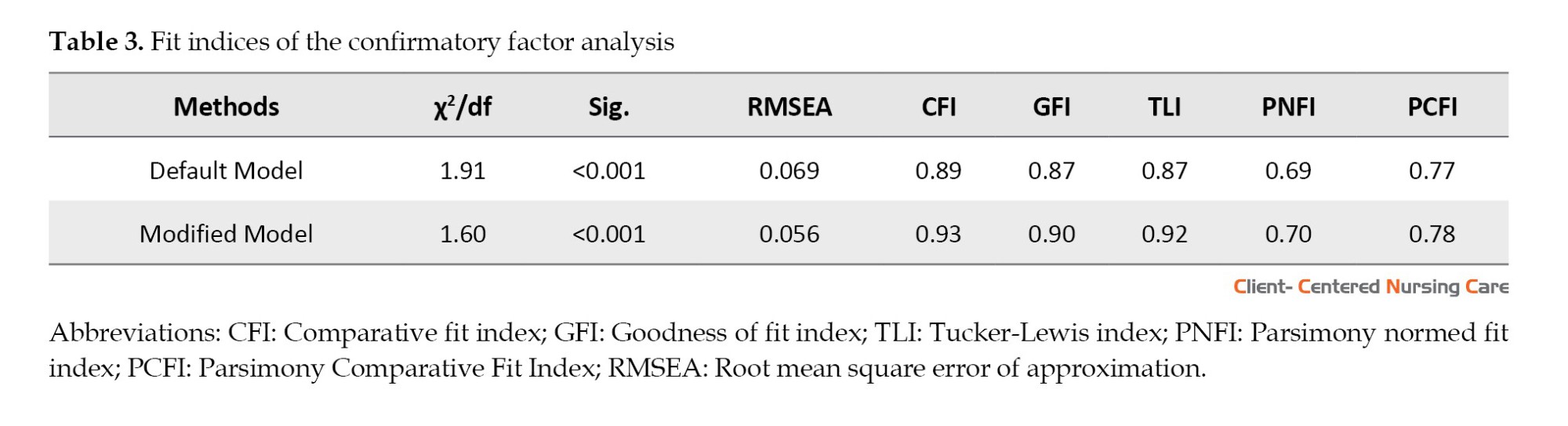
CFA was conducted with 202 participants In the next step to evaluate the 18-item 3 factors scale, using SEM in AMOS software. The factor loading of all questions was more than 0.3 and the indices of the goodness-of-fit were acceptable (χ2/df=1.9, root mean square error of approximation=0.056, comparative fit index=0.93, and parsimony goodness of fit index=0.78). Table 3 demonstrates the model’s good fit, confirming its adequacy.
The obtained values of the fit indices demonstrate that the collected data are adequate for measuring the hidden variables. Therefore, the findings derived from the research model estimation are reliable and trustworthy. Figure 1 illustrates the path analysis related to the questionnaire’s subdomains and their respective fit indices.
Reliability
The calculated Cronbach α reliability coefficient (0.852) and the test re-test assessment of the scale’s consistency and temporal stability (0.862) were within acceptable limits (Table 4).
Discussion
The present study was conducted because of the absence of a suitable tool for measuring the overall impact of living with long-term health conditions among patients with DM. We aimed to establish the psychometric properties of the Persian version of the questionnaire. The LTCQ is a recently developed generic patient-reported outcome measure that captures the experiences of individuals living with long-term conditions. The LTCQ measures different dimensions of a person’s life, including physical and emotional well-being, as well as social participation and self-management skills. Previous studies have shown that the questionnaire is valid for a large and diverse sample of health and social care users (Kelly et al., 2022; Potter et al., 2017).
The present study evaluated the psychometric properties of the questionnaire to ensure the appropriateness and relevance of the instrument, resulting in the retention of all 18 out of 20 items of the questionnaire. The original questionnaire has 3 domains, preserved in the present study. These domains were impact of LTCs, experiences of services and support, and self-care. The final form of the Persian version of LTCQ also has 3 domains: independence (4 items), adaptation to illness (8 items), and self-management (6 items). Although domains in original and Persian version are closely related, the experience of Iranian patients with services and support about DM has not been enough, and then domain of "experiences of services and support» with 2 questions in the original version has changed to domain of «independence» with four questions. The extracted factors in the Persian version of LTCQ are similar to the introduced factors in the original version, and EFA and CFA showed that the questionnaire has an acceptable structural validity and the current scale is a reliable tool to use among Iranian type 2 diabetes patients to assess their overall long-term health conditions.
The domains of the Persian version of LTCQ are very closely related to the domains of Living with long-term condition scale that captures the individual perception of daily living with LTCs (Ambrosio et al., 2021).
The LTCQ assesses how well individuals can manage their daily activities and make decisions about their lives without relying on external support. The independence domain in Persian LTCQ evaluates the extent to which individuals can maintain their autonomy and perform tasks independently, which is crucial for their QOL (Potter et al., 2021). Also, the LTCQ includes items that measure how well individuals have adjusted to their chronic conditions. The domain of adaptation to illness evaluates emotional responses, coping level, and overall well-being, providing insights into how individuals have integrated the experience of illness into their lives (Potter et al., 2017; Potter et al., 2021), and the domain of self-management, including adherence to treatment plans, symptom monitoring, and lifestyle changes (Cundell & McShane, 2023) is another domain in Persian LTCQ that evaluates the individual’s ability to take an active role in managing their health conditions and identify areas where additional support or education may be needed (Cundell & McShane, 2023).
The Coronach α value is 0.95 in the main questionnaire and the initial validation survey has been carried out with over 1200 primary care patients and social care recipients in England (Potter et al., 2017). Besides, in another study, initial validation survey has been conducted as a mixed-method study in England (Potter et al., 2021).
There are more than 15 questionnaires for diabetic patients that assess various aspects of living with diabetes. Some are single-scale, single-dimensional tools, while other tools may consider multiple dimensions of life for diabetic patients. However, none of them fully captures the overall and long-term impacts of diabetes on a diabetic patient’s life (Oluchi et al., 2021). In the current study, we went through standard processes of translation, obtaining face and content validity, doing cross-sectional study, and achieving statistical results in Cronbach α test, ICC, test re-test, EFA, and accessing the goodness of fit indices approved by CFA. It indicates that the instrument is a valid tool for measuring the relevant constructs in the target population. The present research finalized the Persian adaptation of the LTCQ with 18 items and confirmed its validity and reliability through various analyses.
Conclusion
Overall, our study demonstrate that the LTCQ is a valid and reliable instrument for assessing the impacts of living with one or more mental or physical LTCs and is suitable for using in both health and social care settings among patients with DM in Iran. Since data collection relied on self-reports and utilized non-probability sampling methods for participant selection, the sample may not accurately represent the entire population of patients with DM. Therefore, additional studies are required to evaluate the scale’s reliability, stability, and construct validity across various regions. Furthermore, the limited geographical scope of the data collection may restrict the broader applicability of the findings.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran, approved the study (Code: IR.SUMS.NUMIMG.REC.1401.045). The study’s objectives were explained to all the patients, a written consent was obtained, and they were ensured that their information would be kept confidential.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, methodology, validation, and supervision: Zahra Hadian Shirazi, and Marziyeh Momennasab; Software, investigation, resources, and writing: Raziyeh Iloonkashkooli; Formal analysis, data curation, and visualization: Giti Setoodeh, and Mitra Soltanian; Project administration and funding acquisition: Zahra Hadian Shirazi; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank Caroline Potter for granting permission to translate and psychometrically test the LTCQ into Persian. The authors also thank all patients who participated in this study, the staff of Shahid Beheshti Hospital, and all those who assisted them in conducting this research.
References
Diabetes mellitus has become a significant global public health issue. According to the International Diabetes Federation (IDF), 536.6 million people were living with diabetes in 2021, and this figure is expected to rise by 46%, reaching 783.2 million by 2045 (Zan et al., 2024). Approximately 4.2 million deaths among adults aged 20 to 79 are due to diabetes. Globally, diabetes is responsible for an estimated 11.3% of deaths, with nearly half (46.2%) of these occurring in individuals under 60 (Saeedi et al., 2020). Due to its chronic nature, diabetes requires long-term care, continuous monitoring of self-management and support to prevent acute complications and minimize the risk of long-term complications. Health specialists recognize that managing diabetes is intricate and involves addressing numerous factors beyond merely controlling blood sugar levels (Powers et al., 2020).
Not only in Iran, but also in many countries around the world, including Asian countries, the lack of identifying diabetic patients affects the individual’s life, leading to inadequate follow-up and treatment, lacking necessary coordination in disease management, and ultimately significantly increasing diabetes-related mortality (Larijani et al., 2017). Understanding the impact of long-term conditions (LTCs) on the lives of patients with diabetes mellitus (DM) demonstrates the need for multifaceted health interventions and necessitates policymaking to improve treatment by health care providers (Hill-Briggs et al., 2021; Papaspurou et al., 2015). Any health care intervention that can delay the onset of diabetes symptoms or slow the progression of its complications will play a significant role in alleviating patients’ suffering, improving their quality of life (QOL), and reducing the associated costs (Daneshkohan et al., 2019). In this regard, understanding the long-term health conditions of patients with DM is an initial assessment that determines the impact of diabetes on patients’ lives over time and how it deviates them from living in optimal health conditions. Using this knowledge, health care providers will be able to establish long-term care plans for these patients based on the impact of diabetes on their lives, leading to the prevention of complications as much as possible (Ambrosio et al., 2023; Tamornpark et al., 2022).
The LTCs questionnaire (LTCQ) was developed as a patient-reported outcome measure to understand the experience of a satisfactory life with one or more long-term health conditions and multimorbidity (Potter et al., 2017). The questionnaire includes 20 questions that assess the effects of living with one or more long-term mental or physical conditions. It was designed for use in both health and social care contexts. Answers are scored on a scale from 0 (most negative) to 4 (most positive). The scores of all 20 questions are combined into a single overall score, with higher scores reflecting more positive life experiences (Kelly et al., 2022). It is a concise, self-reporting tool to measure the overall impact of long-term conditions (Garcimartin et al., 2017). It was designed to cover both traditional and non-traditional domains that enhance health-related QOL, within a broad concept of “maintaining well-being with LTCs.” The questionnaire complements symptom burden assessments through disease-specific measures. The development of LTCQ involved a series of steps, including literature reviews, stakeholder and public consultation, qualitative interviews with patients, a translatability evaluation, and an initial validation survey (Potter et al., 2021).
The availability of a native questionnaire to evaluate the LTCs of diabetic patients helps health policymakers and health care providers in Iran make informed decisions for health policy and long-term care planning based on the insights gained from these evaluations. Since no study has been conducted in Iran to validate the Persian version of LTCQ, and chronic diseases including diabetes are on the rise, this study aims to perform the cultural adaptation and assess the psychometric properties of the LTCQ in patients with DM.
Materials and Methods
Study design
A methodological study was carried out to evaluate the psychometric properties of LTCQ. It is an instrument to assess ‘living well’ in the context of chronic illness. As a generic patient-reported outcome measure contributing to a wide range of areas across both health care and social services, the LTCQ can meet the distinct need for comprehensive outcome measurement that makes integrated service provision possible. This tool was developed in 2016 by potter and colleagues. The validity and reliability of the questionnaire have been confirmed, with a Cronbach α value of 0.95 and a confidence interval (CI) of 93% to 95%. The questionnaire comprises 20 items, each rated on a 5-point Likert scale ranging from “never=0” to “always=4” with higher scores indicating more favorable living conditions with the disease. The tool’s scores are adjusted to produce a total LTCQ score between 0 and 100 (Potter et al., 2017).
The current study was carried out in two phases. Initially, a forward-backward translation method was applied (Bradley, 2013; WHO, 2023), and the original version of LTCQ was translated into Persian (see Appendix 1). In the second phase, the psychometric properties of the translated instrument were measured.
A cross-sectional study was designed to evaluate the construct validity and reliability of the questionnaire. The subjects were 205 patients with type 2 diabetes selected from the diabetes clinic of Shahid Beheshti Hospital, Shiraz City, Iran, by simple random sampling. The sample size was determined to meet the suggested minimum number of items per case (5-10 items) to ensure the validity of the factor analysis process (Munro, 2005; Yong & Pearce, 2013). The study included individuals with an active profile in the diabetes clinic, aged over 30, and of native Iranian nationality. Participants with major mental disorders, such as Alzheimer, congenital mental retardation, or severe disabilities, like quadriplegia or serious cardiovascular conditions, were excluded. The research team confirmed these criteria to ensure accurate sampling.
Forward-backward translation and cross-cultural adaptation
The instrument was translated from English into Persian following the World Health Organization (WHO) forward-backward translation standard protocol, with prior permission obtained from the corresponding author, as one of the instrument designers (the process of translation and adaptation of instruments, 2009). two fluent English experts in medical sciences were recruited to translate the questionnaire into Persian. After making the necessary corrections in the two translated versions, two other English-speaking experts unaware of the original English text, were requested to translate the Persian version back into English. The resulting translations were compared to each other and to the original text to ensure accuracy in translation. The research team made minor wording and terminology changes in coordination with the translation and re-translation teams. A total of 10 type 2 diabetes patients were interviewed to identify any difficulties they had in reading and understanding the questionnaire. Finally, 10 faculty members verified the cultural relevance of the questionnaire. After preparation of the initial draft of the Persian LTCQ, it was reviewed to ensure its conceptual compatibility with the original version and that the items are fit with the cultural values of Iranian society. Then, the psychometric steps of the questionnaire began.
Qualitative content validity
Content validity is often measured by relying on the knowledge of relevant experts (Zamanzadeh et al., 2014). So, the questionnaire was sent to 10 experts with knowledge and experience in instrument development, adult nursing, psychiatric nursing, and psychology to evaluate the qualitative content validity as described in the translation and cross-cultural adaptation subsection. They were asked to give their opinions about grammar, wording, item allocation, and scaling. After discussion and review by the members, the corrective comments received from the experts were reviewed and implemented.
Quantitative content validity
To evaluate the quantitative content validity of the scale, content validity ratio (CVR) and content validity index (CVI) were determined. In accordance with the Lawshe method, a 3-point scale of ‘necessary,’ ‘useful but not necessary,’ and ‘not necessary’ were utilized to determine CVR, and the items that had a score of at least 0.6 were retained in the questionnaire. To calculate the CVI, the relevance, clarity, and simplicity of each item were assessed on a 4-point Likert scale, and the corresponding index was obtained by dividing the number of experts who chose options 3 and 4 by the total number of experts. Items were accepted based on a CVI score higher than 0.8 and a CVR higher than 0.6 (Polit & Beck, 2006).
Construct validity
In the psychometric evaluation of original questionnaires by its designers, exploratory factor analysis (EFA) is performed to summarize the data, categorize them, and identify its underlying dimensions (Ahmadi et al., 2022; Brown, 2015). As the first step of construct validity assessment, the kurtosis test was performed and data normality was determined among 205 participants (Garson, 2012). Thereafter, the LTCQ factor structure was determined using EFA in SPSS software, version 23. The Kaiser–Meyer–Olkin (KMO) test and Bartlett test for sphericity were used to determine sampling adequacy and appropriateness of the factor analysis. Then, varimax rotation and maximum likelihood model were utilized to identify latent factors and select suitable items. Two items were removed from the factors due to communalities being below 0.3 (Samuels, 2017), and an 18-item questionnaire was resulted. To confirm the result of the EFA and evaluation of the 18-item scale we conducted a CFA, by utilizing structural equation modelling (SEM) in AMOS software, version 24. Therefore, the first- and second-order models were designed and fit indices were reported based on cutoff values (Bollen & Long, 1993).
Reliability
To assess the internal consistency of the questionnaire, the Cronbach α coefficient was calculated. A Cronbach α coefficient range of 0.70 to 0.95 is acceptable (Bland & Altman, 1997). The test re-test reliability assessed the stability of the measurement over time. In this study, the intraclass correlation coefficient (ICC: 0.78 - 0.95) was utilized to evaluate test re-test reliability. An ICC score above 0.80 was deemed acceptable (Koo & Li, 2016; Anselmi et al., 2019).
Results
The Persian version of the tool was developed through a forward-backward translation procedure conducted by four experts fluent in English. Ten patients with diabetes were also interviewed to ensure understanding of the content of the tool. Ultimately, the Persian version of the tool was developed for the evaluation of its psychometric properties.
Content and face validity
In the qualitative section, some health-related terms not commonly used in Iran were substituted with equivalent terms more widely recognized and accepted in our country. For example, the expression “despite health conditions” changed to “despite the disease” in some instances. This revision was done to ensure that the study materials were accessible and easily understood by all participants, regardless of their level of familiarity with medical terminology.
As mentioned before, evaluation of content validity involved a thorough consultation process with experts in the qualitative section. The experts’ suggestions were taken into account to enhance the questionnaire’s content validity, resulting in the modification of the instrument. Regarding quantitative content validity, all items in the questionnaire were analyzed, and the CVR scores for all items surpassed 0.6, while the CVI scores were greater than 0.8. The results indicated that the questionnaire had a high level of content validity and none of the items needed to be removed, demonstrating the suitability of the questionnaire.
Construct validity
To examine construct validity, we carried out a cross-sectional study, involving a sample of 205 patients. Of these patients, over half were female (53.7%). The age of the patients ranged from 20 to 68 years, with a Mean±SD age of 48.3±18.4 years. Moreover, 84.7% of participants were individuals without a university degree, and 15.3% had some university education. The mean duration of diabetes among the participants was 7.9±5.6 years, ranging from 2 to 14 years. It was noted that the majority of the participants (72.6%) had at least one complication associated with diabetes. These demographic characteristics provided valuable insights into the study sample and facilitated the interpretation of study results (Table 1).

Before performing factor analysis, the initial 20-item questionnaire demonstrated a Cronbach α value of 0.852, indicating a high level of internal consistency. Using the kurtosis test, it was determined that all the items fell within the acceptable range of -1 to 1, and therefore, no items were removed. Furthermore, the normality test and boxplot analysis identified 3 participants (45, 48, and 197) as consistent outliers across multiple items. These participants were subsequently excluded from the dataset. Regarding the results of EFA, good results of Bartlett test of sphericity (χ2=1386.523; P<0.001) and the KMO (0.85) showed sampling adequacy and provided minimum standards for conducting a factor analysis (Bucci et al., 2018; Field, 2013). Having excluded 3 participants, we proceeded with EFA, anticipating a three-factor structure. We assessed item suitability under these factors, eliminating items with loadings below 0.30. After utilizing varimax rotation, the maximum likelihood model demonstrated superior statistical and theoretical adequacy (Wood et al., 1996). In particular, items 12 and 20 were omitted from the analysis due to being inadequately fit. EFA extracted 3 factors. Using a panel of professionals in health promotion and psychology, we named these 3 factors. They were categorized as independence, adaptation to illness, and self-management. Main factors of LTCQ are presented in Table 2.

CFA was conducted with 202 participants In the next step to evaluate the 18-item 3 factors scale, using SEM in AMOS software. The factor loading of all questions was more than 0.3 and the indices of the goodness-of-fit were acceptable (χ2/df=1.9, root mean square error of approximation=0.056, comparative fit index=0.93, and parsimony goodness of fit index=0.78). Table 3 demonstrates the model’s good fit, confirming its adequacy.

The obtained values of the fit indices demonstrate that the collected data are adequate for measuring the hidden variables. Therefore, the findings derived from the research model estimation are reliable and trustworthy. Figure 1 illustrates the path analysis related to the questionnaire’s subdomains and their respective fit indices.

Reliability
The calculated Cronbach α reliability coefficient (0.852) and the test re-test assessment of the scale’s consistency and temporal stability (0.862) were within acceptable limits (Table 4).

Discussion
The present study was conducted because of the absence of a suitable tool for measuring the overall impact of living with long-term health conditions among patients with DM. We aimed to establish the psychometric properties of the Persian version of the questionnaire. The LTCQ is a recently developed generic patient-reported outcome measure that captures the experiences of individuals living with long-term conditions. The LTCQ measures different dimensions of a person’s life, including physical and emotional well-being, as well as social participation and self-management skills. Previous studies have shown that the questionnaire is valid for a large and diverse sample of health and social care users (Kelly et al., 2022; Potter et al., 2017).
The present study evaluated the psychometric properties of the questionnaire to ensure the appropriateness and relevance of the instrument, resulting in the retention of all 18 out of 20 items of the questionnaire. The original questionnaire has 3 domains, preserved in the present study. These domains were impact of LTCs, experiences of services and support, and self-care. The final form of the Persian version of LTCQ also has 3 domains: independence (4 items), adaptation to illness (8 items), and self-management (6 items). Although domains in original and Persian version are closely related, the experience of Iranian patients with services and support about DM has not been enough, and then domain of "experiences of services and support» with 2 questions in the original version has changed to domain of «independence» with four questions. The extracted factors in the Persian version of LTCQ are similar to the introduced factors in the original version, and EFA and CFA showed that the questionnaire has an acceptable structural validity and the current scale is a reliable tool to use among Iranian type 2 diabetes patients to assess their overall long-term health conditions.
The domains of the Persian version of LTCQ are very closely related to the domains of Living with long-term condition scale that captures the individual perception of daily living with LTCs (Ambrosio et al., 2021).
The LTCQ assesses how well individuals can manage their daily activities and make decisions about their lives without relying on external support. The independence domain in Persian LTCQ evaluates the extent to which individuals can maintain their autonomy and perform tasks independently, which is crucial for their QOL (Potter et al., 2021). Also, the LTCQ includes items that measure how well individuals have adjusted to their chronic conditions. The domain of adaptation to illness evaluates emotional responses, coping level, and overall well-being, providing insights into how individuals have integrated the experience of illness into their lives (Potter et al., 2017; Potter et al., 2021), and the domain of self-management, including adherence to treatment plans, symptom monitoring, and lifestyle changes (Cundell & McShane, 2023) is another domain in Persian LTCQ that evaluates the individual’s ability to take an active role in managing their health conditions and identify areas where additional support or education may be needed (Cundell & McShane, 2023).
The Coronach α value is 0.95 in the main questionnaire and the initial validation survey has been carried out with over 1200 primary care patients and social care recipients in England (Potter et al., 2017). Besides, in another study, initial validation survey has been conducted as a mixed-method study in England (Potter et al., 2021).
There are more than 15 questionnaires for diabetic patients that assess various aspects of living with diabetes. Some are single-scale, single-dimensional tools, while other tools may consider multiple dimensions of life for diabetic patients. However, none of them fully captures the overall and long-term impacts of diabetes on a diabetic patient’s life (Oluchi et al., 2021). In the current study, we went through standard processes of translation, obtaining face and content validity, doing cross-sectional study, and achieving statistical results in Cronbach α test, ICC, test re-test, EFA, and accessing the goodness of fit indices approved by CFA. It indicates that the instrument is a valid tool for measuring the relevant constructs in the target population. The present research finalized the Persian adaptation of the LTCQ with 18 items and confirmed its validity and reliability through various analyses.
Conclusion
Overall, our study demonstrate that the LTCQ is a valid and reliable instrument for assessing the impacts of living with one or more mental or physical LTCs and is suitable for using in both health and social care settings among patients with DM in Iran. Since data collection relied on self-reports and utilized non-probability sampling methods for participant selection, the sample may not accurately represent the entire population of patients with DM. Therefore, additional studies are required to evaluate the scale’s reliability, stability, and construct validity across various regions. Furthermore, the limited geographical scope of the data collection may restrict the broader applicability of the findings.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran, approved the study (Code: IR.SUMS.NUMIMG.REC.1401.045). The study’s objectives were explained to all the patients, a written consent was obtained, and they were ensured that their information would be kept confidential.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, methodology, validation, and supervision: Zahra Hadian Shirazi, and Marziyeh Momennasab; Software, investigation, resources, and writing: Raziyeh Iloonkashkooli; Formal analysis, data curation, and visualization: Giti Setoodeh, and Mitra Soltanian; Project administration and funding acquisition: Zahra Hadian Shirazi; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank Caroline Potter for granting permission to translate and psychometrically test the LTCQ into Persian. The authors also thank all patients who participated in this study, the staff of Shahid Beheshti Hospital, and all those who assisted them in conducting this research.
References
Ahmadi, A., Niknami, S. & Ghaffari, M., 2022. Type 2 Diabetes Health Literacy Assessment Tool: Translation and psychometric evaluation of the Iranian Version. International Journal of Endocrinology and Metabolism, 20(2), pp.e116983. [DOI:10.5812/ijem-116983] [PMID]
Ambrosio, L., et al., 2023. First validation study of the living with long term conditions scale (LwLTCs) among English-speaking population living with Parkinson’s disease. Health and Quality of Life Outcomes, 21(1), pp. 69. [DOI:10.1186/s12955-023-02154-6] [PMID]
Ambrosio, L., et al., 2021. Living with Long term condition Scale: A pilot validation study of a new person centred tool in the UK. Nursing Open, 8(4), pp. 1909–19. [DOI:10.1002/nop2.859] [PMID]
Anselmi, P., Colledani, D. & Robusto, E., 2019. A comparison of classical and modern measures of internal consistency. Frontiers in Psychology, 10, 2714. [DOI:10.3389/fpsyg.2019.02714] [PMID]
Bland, J. M. & Altman, D. G., 1997. Cronbach’s alpha. BMJ (Clinical research ed.), 314(7080), pp. 572. [DOI:10.1136/bmj.314.7080.572] [PMID]
Bollen, K. A. & Long, J. S., 1993. Testing structural equation models. Newbury Park: Sage. [Link]
Bradley, C., 2013. Translation of questionnaires for use in different languages and cultures. In C. Bradley (Ed.), Handbook of psychology and diabetes (pp. 43-55). London: Routledge. [DOI:10.4324/9781315077369]
Brown, T. A., 2015. Confirmatory factor analysis for applied research. New York: Guilford Publications. [Link]
Bucci, N., et al., 2018. Factor analysis of the psychosocial risk assessment instrument. Data Mining and Big Data: Third International Conference, DMBD 2018, Shanghai, China, June 17-22, 2018. [Link]
Daneshkohan, A., et al., 2019. [The relationship of quality of services provided to type 2 Diabetic patients with self-assessment in Comprehensive Health Service Centers (Persian)]. Journal of Health Based Research, 5(2), pp. 187-201. [DOI: 10.22062/5.2.187]
Field, A., 2013. Discovering statistics using IBM SPSS statistics. California: Sage. [Link]
Garcimartin, P., et al., 2017. Transcultural adaptation and validation of the patient empowerment in long-term conditions questionnaire. BMC Health Services Research, 17(1), pp. 324. [DOI:10.1186/s12913-017-2271-7] [PMID]
Garson, D., 2012. Testing statistical assumptions. Asheboro: Statistical Associates Publishing. [Link]
Hill-Briggs, F., et al., 2021. Social determinants of health and diabetes: A scientific review. Diabetes Care, 44(1), pp. 258–79. [DOI:10.2337/dci20-0053] [PMID]
Kelly, L., et al., 2022. Assessing the validity of the Long-Term Conditions Questionnaire (LTCQ) in women during pregnancy and the first year following birth. Patient Related Outcome Measures, 13, pp. 221–8. [DOI:10.2147/PROM.S376070] [PMID]
Koo, T. K. & Li, M. Y., 2016. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15(2), pp. 155–63. [DOI:10.1016/j.jcm.2016.02.012] [PMID]
Larijani,B., et al., 2017. [Iranian National Service Framework for Diabetes (Persian)]. Tehran: National Committee for Control and Prevention of Noncommunicable Diseases.
Munro, B. H., 2005. Statistical methods for health care research (Vol. 1). Pennsylvania: lippincott williams & wilkins. [Link]
Oluchi, S. E., et al., 2021. Health related quality of life measurements for diabetes: A systematic review. International Journal of Environmental Research and Public Health, 18(17), pp. 9245. [DOI:10.3390/ijerph18179245] [PMID]
Papaspurou, M., et al., 2015. Fears and health needs of patients with Diabetes: A qualitative research in rural population. Medical Archives (Sarajevo, Bosnia and Herzegovina), 69(3), pp. 190–5. [DOI:10.5455/medarh.2015.69.190-195] [PMID]
Polit, D. F. & Beck, C. T., 2006. The content validity index: Are you sure you know what’s being reported? Critique and recommendations. Research in Nursing & Health, 29(5), pp. 489–97. [DOI:10.1002/nur.20147] [PMID]
Potter, C. M., et al., 2017. Long-Term Conditions Questionnaire (LTCQ): Initial validation survey among primary care patients and social care recipients in England. BMJ Open, 7(11), pp. e019235. [DOI:10.1136/bmjopen-2017-019235] [PMID]
Potter, C. M., et al., 2023. Living well while providing support: Validation of LTCQ-Carer for assessing informal carers' quality of life. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 32(12), pp. 3507–20. [DOI:10.1007/s11136-023-03485-z] [PMID]
Potter, C. M., et al., 2021. Use of the Long-Term Conditions Questionnaire (LTCQ) for monitoring health-related quality of life in people affected by cognitive impairment including dementia: pilot study in UK memory clinic services. Quality of Life Research, 30(6), pp. 1641–52. [DOI:10.1007/s11136-021-02762-z] [PMID]
Powers, M. A., et al., 2020. Diabetes Self-management Education and Support in Adults With Type 2 Diabetes: A Consensus Report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. Journal of the American Pharmacists Association: JAPhA, 60(6), pp. e1–18. [DOI:10.1016/j.japh.2020.04.018] [PMID]
Saeedi, P., et al., 2020. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice, 162, pp. 108086. [DOI:10.1016/j.diabres.2020.108086] [PMID]
Samuels, P., 2017. Advice on exploratory factor analysis. Birmingham: Birmingham City University. [Link]
Tamornpark, R., et al., 2022. Quality of life and factors associated with a good quality of life among diabetes mellitus patients in northern Thailand. Health and Quality of Life Outcomes, 20(1), pp. 81. [DOI:10.1186/s12955-022-01986-y] [PMID]
Wood, J. M., Tataryn, D. J. & Gorsuch, R. L., 1996. Effects of under-and overextraction on principal axis factor analysis with varimax rotation. Psychological Methods, 1(4), pp. 354-65. [DOI:10.1037/1082-989X.1.4.354]
World Health Organization., 2023. Adaptation and translation guide. Geneva: World Health Organization. [Link]
Yong, A. G. & Pearce, S., 2013. A beginner’s guide to factor analysis: Focusing on exploratory factor analysis. Tutorials in Quantitative Methods for Psychology, 9(2), pp. 79-94. [DOI:10.20982/tqmp.09.2.p079]
Zamanzadeh, V., et al., 2015. Details of content validity and objectifying it in instrument development. Nursing Practice Today, 1(3), pp. 163-71. [Link]
Type of Study: Research |
Subject:
Special
Received: 2024/11/20 | Accepted: 2025/02/18 | Published: 2025/05/1
Received: 2024/11/20 | Accepted: 2025/02/18 | Published: 2025/05/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |