Thu, Jan 8, 2026
[Archive]
Volume 11, Issue 1 (Winter 2025)
JCCNC 2025, 11(1): 55-66 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hadipour S, Amini L, Afshar B, Ghasemi A, Haghani H, Sadeghi Avval Shahr H et al . The Effects of Cinnamon on Metabolic and Hormonal Status of Women With Polycystic Ovary Syndrome: A Randomized Controlled Trial. JCCNC 2025; 11 (1) :55-66
URL: http://jccnc.iums.ac.ir/article-1-626-en.html
URL: http://jccnc.iums.ac.ir/article-1-626-en.html
Sanam Hadipour1 

 , Leila Amini *2
, Leila Amini *2 

 , Bahare Afshar1
, Bahare Afshar1 

 , Afsaneh Ghasemi3
, Afsaneh Ghasemi3 

 , Hamid Haghani4
, Hamid Haghani4 

 , Homa Sadeghi Avval Shahr5
, Homa Sadeghi Avval Shahr5 

 , Shayesteh Jahanfar6
, Shayesteh Jahanfar6 




 , Leila Amini *2
, Leila Amini *2 

 , Bahare Afshar1
, Bahare Afshar1 

 , Afsaneh Ghasemi3
, Afsaneh Ghasemi3 

 , Hamid Haghani4
, Hamid Haghani4 

 , Homa Sadeghi Avval Shahr5
, Homa Sadeghi Avval Shahr5 

 , Shayesteh Jahanfar6
, Shayesteh Jahanfar6 


1- Department of Midwifery and Reproductive Health, School of Nursing and Midwifery, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Midwifery and Reproductive Health, School of Nursing and Midwifery, Iran University of Medical Sciences, Tehran, Iran. ,amini.l@iums.ac.ir
3- Clinical Research Development Unit, Shahid Akbar Abadi Hospital, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
4- Department of Biostatistics, School of Public Health, Iran University of Medical Sciences, Tehran, Iran.
5- Department of Midwifery and Reproductive, School of Nursing and Midwifery, Iran University of Medical Sciences, Tehran, Iran.
6- Department of Public Health and Community Medicine, Tufts University School of Medicine, Boston, United States.
2- Department of Midwifery and Reproductive Health, School of Nursing and Midwifery, Iran University of Medical Sciences, Tehran, Iran. ,
3- Clinical Research Development Unit, Shahid Akbar Abadi Hospital, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
4- Department of Biostatistics, School of Public Health, Iran University of Medical Sciences, Tehran, Iran.
5- Department of Midwifery and Reproductive, School of Nursing and Midwifery, Iran University of Medical Sciences, Tehran, Iran.
6- Department of Public Health and Community Medicine, Tufts University School of Medicine, Boston, United States.
Full-Text [PDF 725 kb]
(2452 Downloads)
| Abstract (HTML) (2288 Views)
Full-Text: (1033 Views)
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age. The prevalence of PCOS, according to Rotterdam criteria, is about 6% to 18% (Blagojevic et al., 2017). The prevalence of this disease in various studies conducted in Iran has been reported from 7% to 19.4% (Farhadi-Azar et al., 2022; Ghiasi, 2019). PCOS has received a lot of attention due to its high prevalence and possible consequences such as infertility, and also metabolic and cardiovascular disorders in women. The exact cause of PCOS is unknown; however, it has currently been confirmed that its occurrence is due to genetic and environmental factors (Boyle & Teede, 2012). In addition, some environmental factors, such as diet, physical activity, smoking and stress can also be involved in causing this syndrome (Michalak et al., 2015). Although compensatory insulin resistance and hyperinsulinemia are not diagnostic criteria for PCOS but its prevalence among women with this syndrome is 50%-70% and may reach 95% in overweight women (Qin et al., 2010). PCOS is also the most common cause of infertility, such that 50%-70% of women with infertility due to anovulation have this syndrome (Nasir Amiri et al., 2013). These have made PCOS a frustrating experience for women and a complex scientific challenge for researchers and physicians (Johnson, 2014).
To reduce and treat the physical complications of PCOS and promote the health of women with this syndrome, many pharmacological and non-pharmacological methods have been considered and studied. Complementary therapies in these women have increased over the past 10 years (Smith et al., 2013). One of the common types of alternative drugs is herbal medicine such as saliva officinalis (Amini et al., 2020), curcumin, bebeerine (Joshi et al., 2021), and cinnamon (Baker et al., 2008). Herbal medicines have fewer side effects than conventional treatments (Nagarathna et al., 2014). Cinnamon is a low-risk medicinal plant used daily without any side effects (Rao & Gan, 2014). The use of cinnamon as a potentially useful drug for the treatment of type 2 diabetes began almost about 20 years ago (Sangal, 2011). It has been shown during laboratory and animal studies that cinnamon stimulates insulin secretion (Broadhurst et al., 2000) and cinnamon extract increases the activity of insulin kinase receptors by increasing the activity of phosphatidylinositol 3-kinases (PI3K) in the insulin signaling pathway in the cell, resulting in improved insulin function and increased blood glucose uptake (Heibashy et al., 2013). A study showed the ability of cinnamon to reduce lipid levels in fructose-fed mice (El-Bidawy et al., 2014). The effect of these compounds is also positive in reducing TGs, cholesterol, and low-density lipoprotein (Modaresi et al., 2009). However, another study showed that daily intake of 1 g of cinnamon for three months did not cause significant changes in fasting glucose and glycosylated hemoglobin (Blevins et al., 2007a). Despite several studies that have examined the effects of cinnamon on PCOS aspects, there are controversies in this regard. Accordingly, this study determines the effect of oral cinnamon on metabolic profile in women with PCOS.
Materials and Methods
Study design, setting, and sample
This study was a triple-blinded randomized placebo-controlled clinical trial with parallel groups and was performed on 66 women with PCOS referred to the gynecology and Infertility Clinic of Firoozgar Hospital affiliated with Iran University of Medical Sciences and some selected private centers in Tehran City, Iran, from June 2016 to November 2017 to investigate cinnamon effects on the metabolic status of women with PCOS.
The sample size was calculated based on fasting blood sugar (FBS) mean differences between cinnamon and placebo groups using the Equation 1. It was assumed that a total sample size of 66 subjects (33 participants in each group), would provide an 80% power with a type I error of 0.05 to detect a statistically significant difference in FBS mean between the two studied groups.
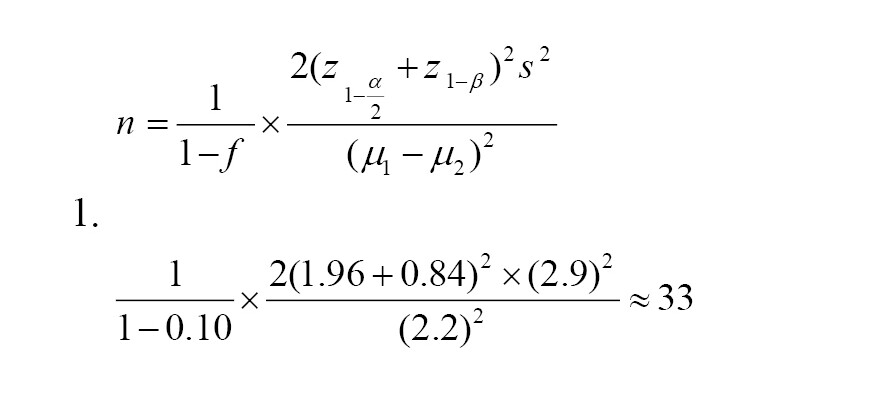
Iranian women aged 18-40 years, with a definitive diagnosis of PCOS by an expert gynecologist, were included in the study according to Rotterdam criteria. The Rotterdam criteria require the presence of two of the following symptoms in the patient: a) Oligo-ovulation or anovulation; b) Hyperandrogenism clinical (such as hirsutism) or biological (including increased free androgen or free testosterone) signs; and c) Polycystic ovaries in ultrasound view (Wang & Mol, 2017). Hirsutism defined as a Ferriman-Gallwey score more than 8 (Ferriman & Gallwey, 1961). In this study, all the women who have used other herbal remedies, glucose or lipid metabolism-affecting drugs, vegetarians, women with body mass index under 19 or over 35 kg/m2, patients with metabolic disorders, and pregnant or nursery women were excluded. Finally, out of 113 participants assessed, 81 women were eligible for the trial and signed an informed consent form. The participants were randomly allocated to cinnamon (n=41) or placebo (n=40) groups by using a randomization list. Randomization was performed by a person who was not involved in the research process. The main outcome measure was metabolic status including FBS, triglyceride (TG), low density cholesterol (LDL and LDL-C), high density cholesterol (HDL and HDL-C), total cholesterol, sex hormone binding globulin (SHBG), total testosterone (TT), fasting insulin (FI), and free androgen index (FAI).
Study measurements
All the subjects completed a demographic and fertility questionnaire, which included information such as age, marital status, duration of education, occupational status, economic status, age of menarche, and body mass index (BMI) using the self-report method before the intervention. Blood test results were recorded before and 12 weeks after intervention by the researcher in a data sheet based on laboratory test reports. All blood samples were collected after 12-14 h of overnight fasting. After taking blood, the samples were placed at room temperature for 20-30 min and then were centrifuged at 2500-3500 revolutions per minute (rpm) for 10-15 min. Blood sera were isolated and stored at -80 ºC for later assessments. All the samples were analyzed in the laboratory of the research in the Laboratory of Gynecology and Infertility Clinic of Firoozgar Hospital. FBS was measured in milligrams per deciliter (mg/dL), TG (mg/dL), and total cholesterol (mg/dL) levels were determined by Pars Azmoon Company enzymatic kits (Tehran, Iran) and Hitachi 912 auto-analyzer (Japan), and levels of LDL-C (mg/dl) and HDL-C (mg/dL) were determined by Pishtaz Teb Company kit (Tehran, Iran) and Hitachi 912 auto-analyzer (Japan). Also, the FI (milli-international units per liter [mIU/L]) was measured by enzyme-linked immunosorbent assay method using the commercial kit (Insulin: Monobind Inc., Lake Forest, CA, USA) with an automated microplate reader (Hyperion Inc., USA). FAI was calculated to determine the androgen status of the subjects using the Equation 2:
2. FAI=(100×total TT [nanomoles per liter or nmol/l])/SHBG (nmol/L)
Also, BMI was calculated using the Equation 3:
3. BMI (kg/m2)=weight (kg)/ height2 [m]
Variables were measured twice, once before the intervention and once 12 weeks after the onset of the intervention.
Preparations of the cinnamon extract capsules and the placebo
The cinnamon was taken from an herbal market in Tehran City, Iran, and was approved by a pharmacognosy expert at the School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The appropriate dosage was selected based on the recommended dosage in Iranian Traditional Medicine and physician’s desk reference for herbal medicines. According to the mentioned references, the average dose of 3 g/day was selected. In the next step, cinnamon was ground using an electrical grinder, and the resulting powder was combined with 96º ethanol as the solvent. After the solvent evaporation, the remaining extract was mixed with corn starch and turned into a powder. A total of 3 g of that powder then were placed into each size 0 capsule manually. For the placebo, the same capsules were filled with corn starch alone. Finally, the capsules were packaged and coded in similar boxes and named A or B by a person independent of the research team. All of the participants, researchers, and statisticians were blinded to the groups.
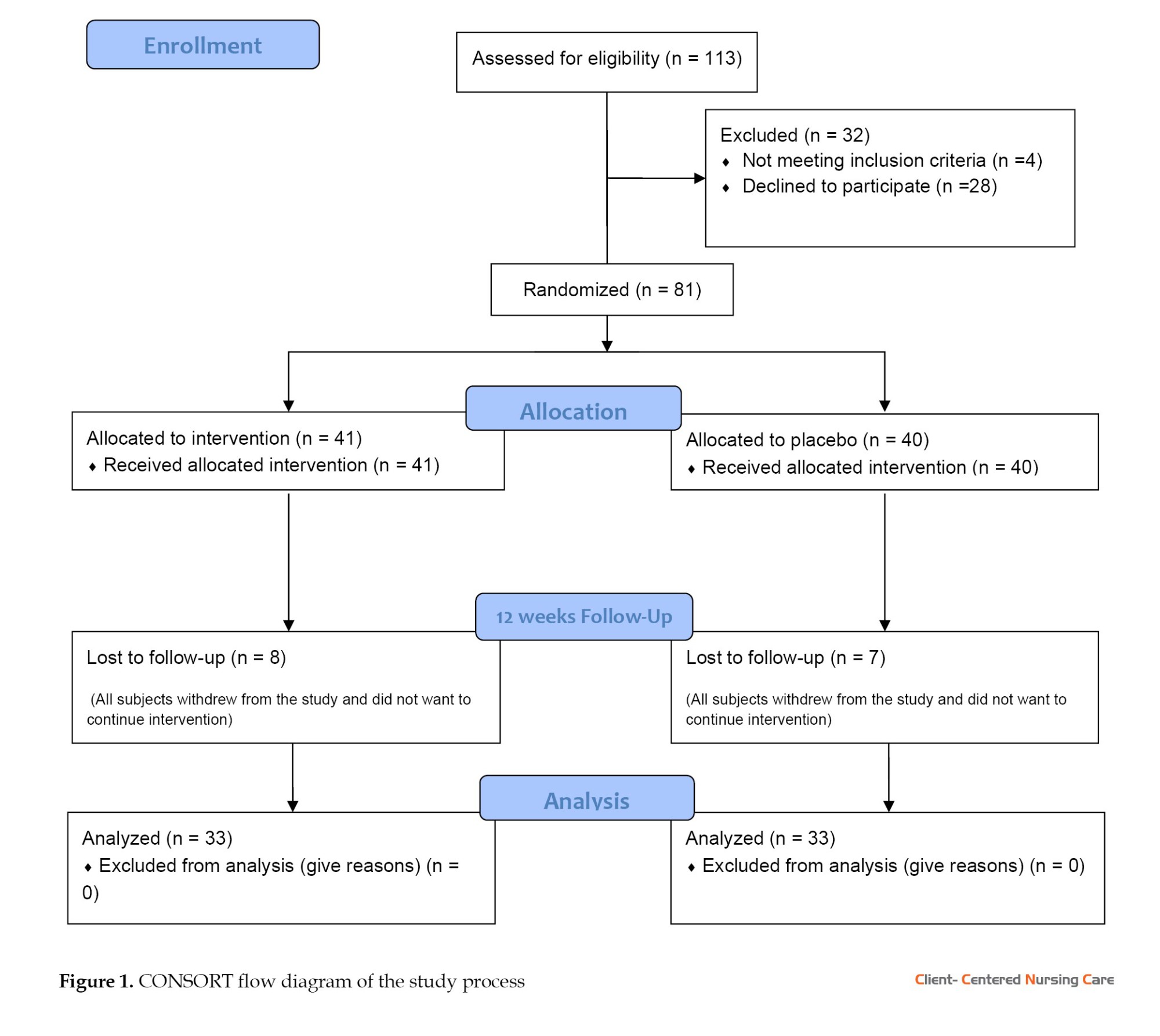
After initial measurements, intervention was started. Some of the subjects in both groups withdrew during the follow-up (Figure 1). Participants in the cinnamon group (n=33) took the cinnamon extract capsules 3 g/day orally for twelve consecutive weeks, and the placebo group (n=33) simultaneously and in parallel took placebo capsules orally once a day for twelve consecutive weeks. During the study period, the subjects were monitored by telephone once or twice a week and were asked to report any drug side effects, or complications, or stop taking the drug. Women who did not take more than 2 capsules for any reason, were excluded from the study (Figure 1).
Statistical analysis
The normal distribution of the data was checked by the Kolmogorov-Smirnov test and graphical methods. Data was presented as Mean±SD for quantitative data or frequency and percent (for qualitative data) in tables. The Student t-test was used for between-group comparison of quantitative variables with normal distribution, while the paired t-test was used to compare them before and after the intervention in each group. The Mann-Whitney U test was used for between-group comparison of quantitative variables without a normal distribution. Categorical variables were compared using the chi-square test. Analysis of covariance was used when there were significant differences between the groups before the intervention. All statistical analyses were performed using the SPSS software, version 16 (SPSS Inc., Chicago, IL, USA). Meanwhile, the significance level was set at P<0.05.
Results
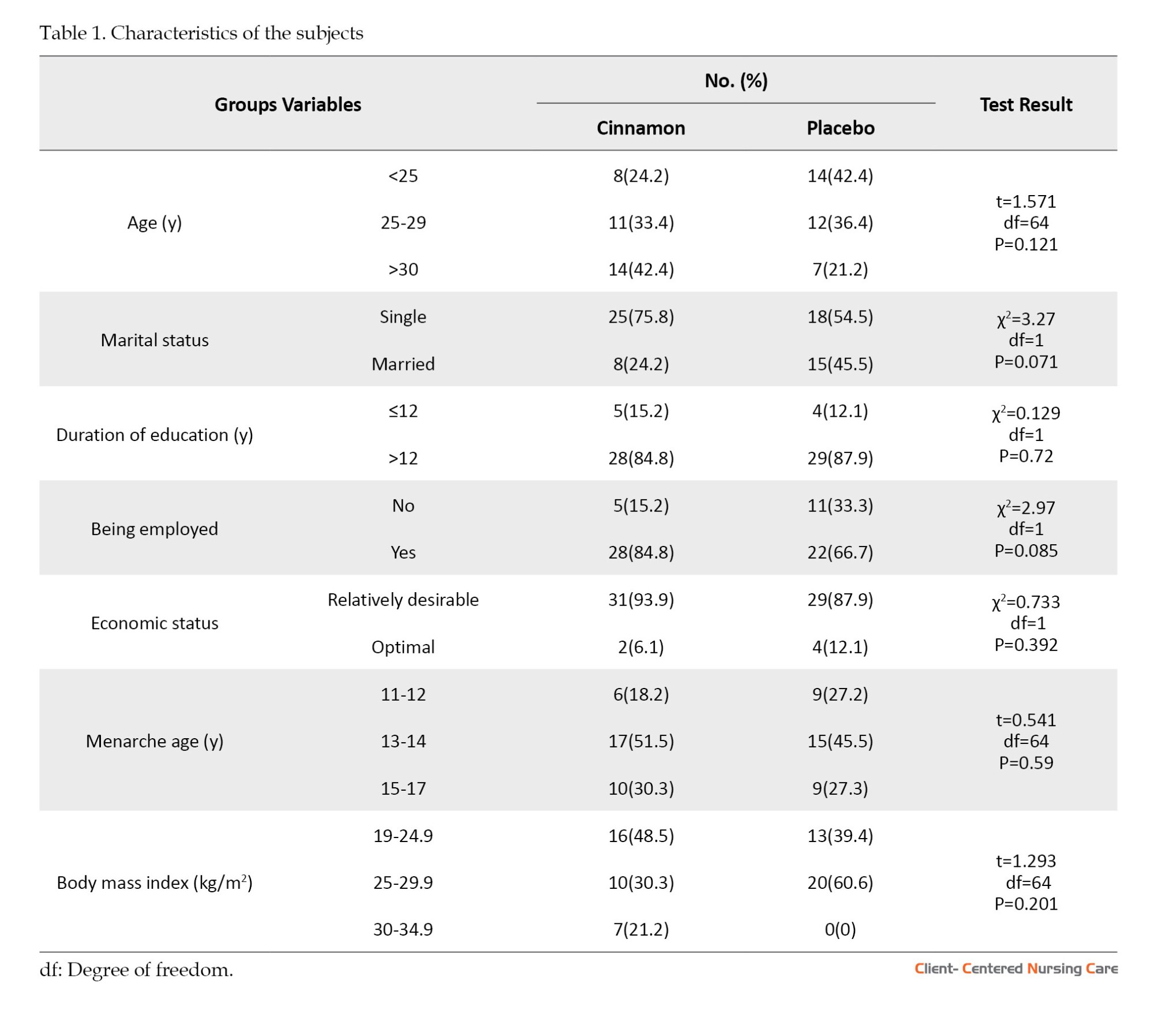
The mean age of the subjects was 27.4±78.9 and 26.31±4.0 in the intervention and control groups, respectively. The characteristics of the subjects are summarized in Table 1. Accordingly, the random allocation could balance between the two groups in terms of the demographic and reproductive characteristics of the participants. Therefore, there were no statistically significant differences between the groups regarding these variables.
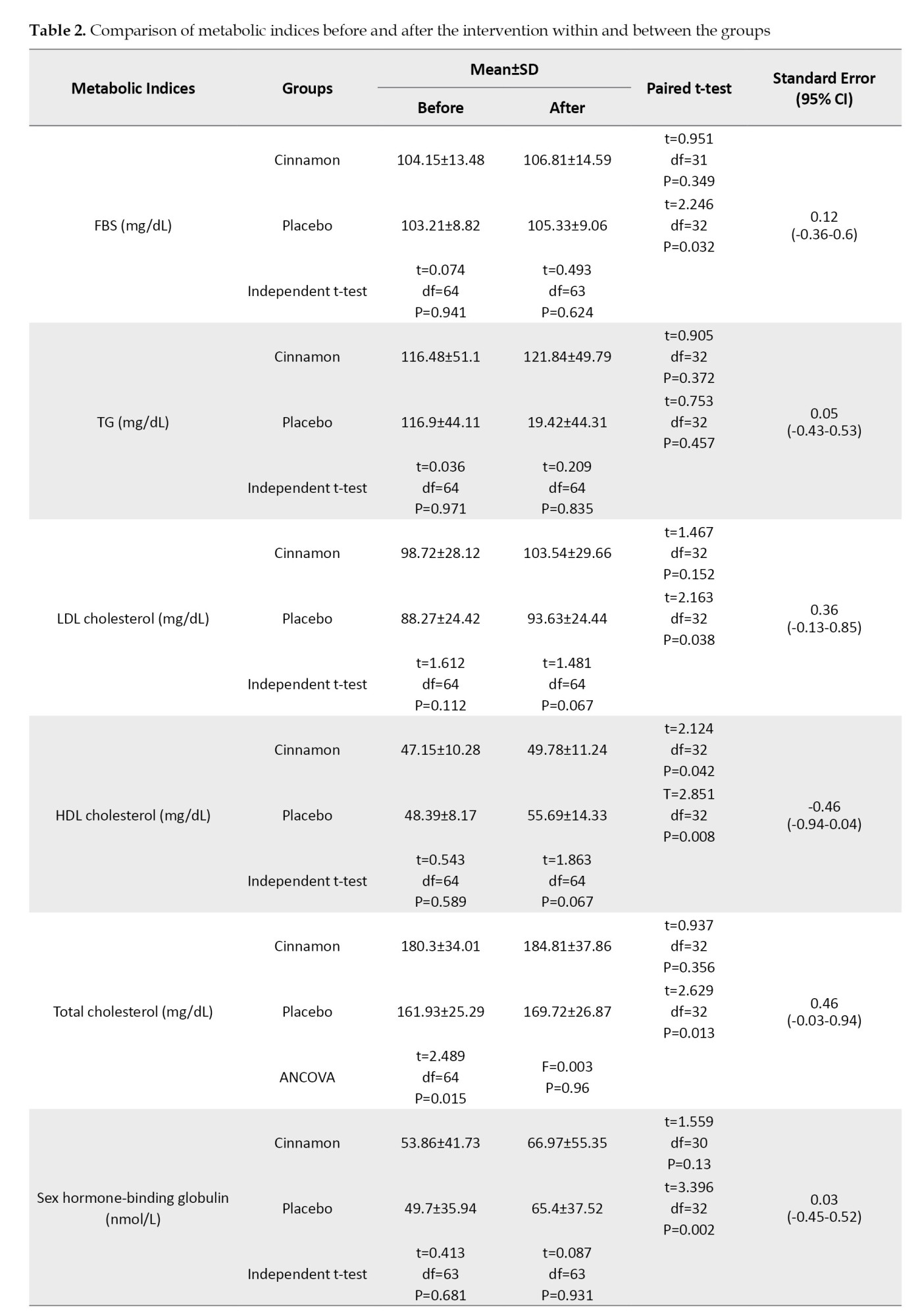
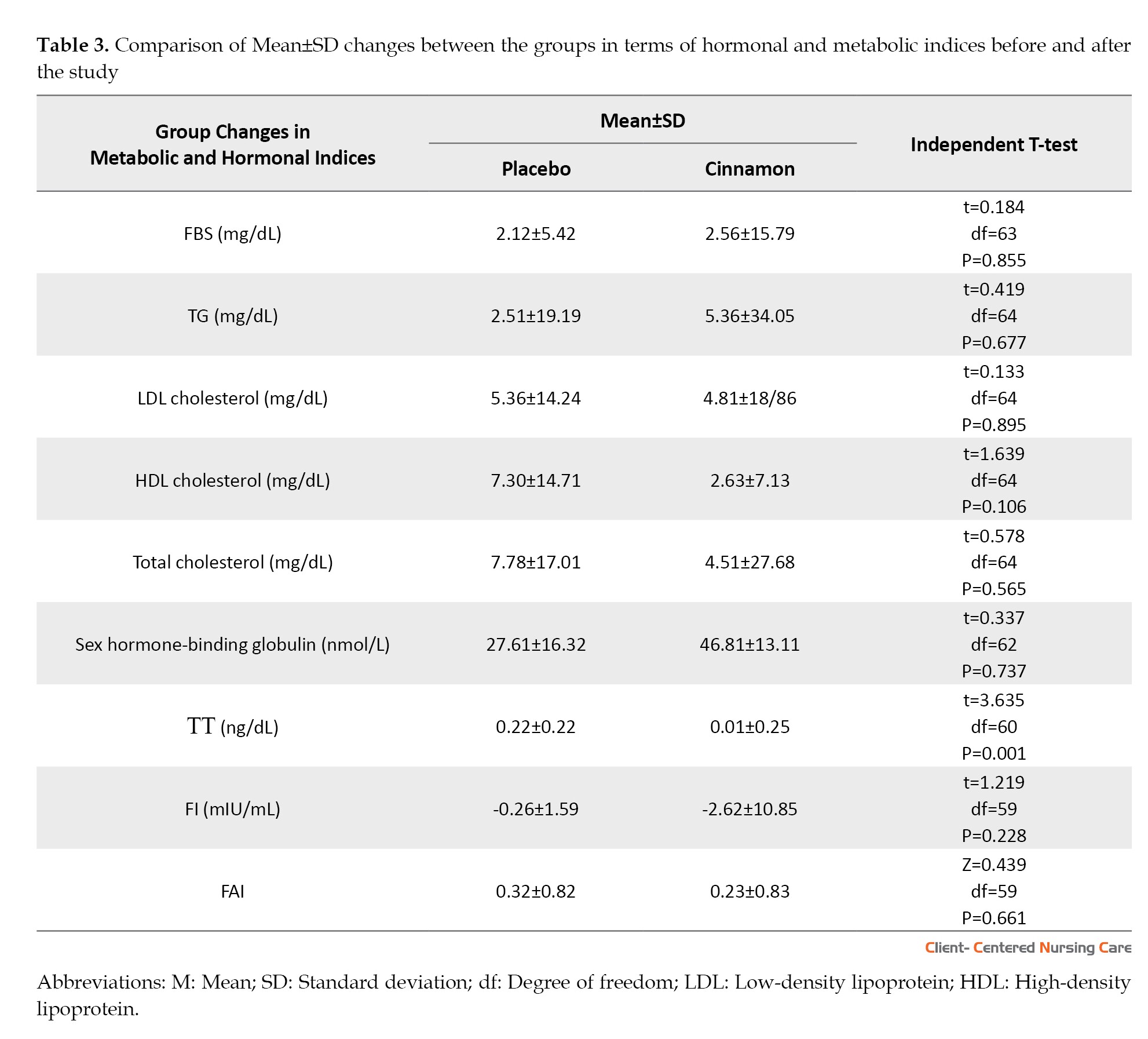
The results of the independent t-test in Table 2 show that there are no significant differences between the groups before intervention regarding FBS, TG, LDL-C, HDL-C, SHBG, TT, and FAI; however, total cholesterol (P=0.015) and FI (P=0.003) were statistically lower in the placebo group than the cinnamon group. At the end of the study, the two groups did not show any significant differences in terms of FBS, TG, LDL-C, HDL-C, total cholesterol, SHBG, and FAI; however, the women in the cinnamon group had a significantly lower TT than other group (P=0.001). Also, the results of the paired t-test showed that in the placebo group, FBS, LDL-C, HDL-C, total cholesterol, TT, SHBG, and FAI had a significant increase at the end of the study (P<0.05); however, TG and FI did not show any statistical changes. In the cinnamon group, only HDL-C showed a significant increase after the intervention compared to before (P=0.042), and the other hormones and metabolic indicators remained unchanged. Since total cholesterol and FI were significantly lower in the placebo group compared to the cinnamon group, the analysis of covariance was used to compare the mentioned variables between the two groups at the end of the study. The results showed that there were no significant differences between the two groups regarding total cholesterol and FI (Table 2).
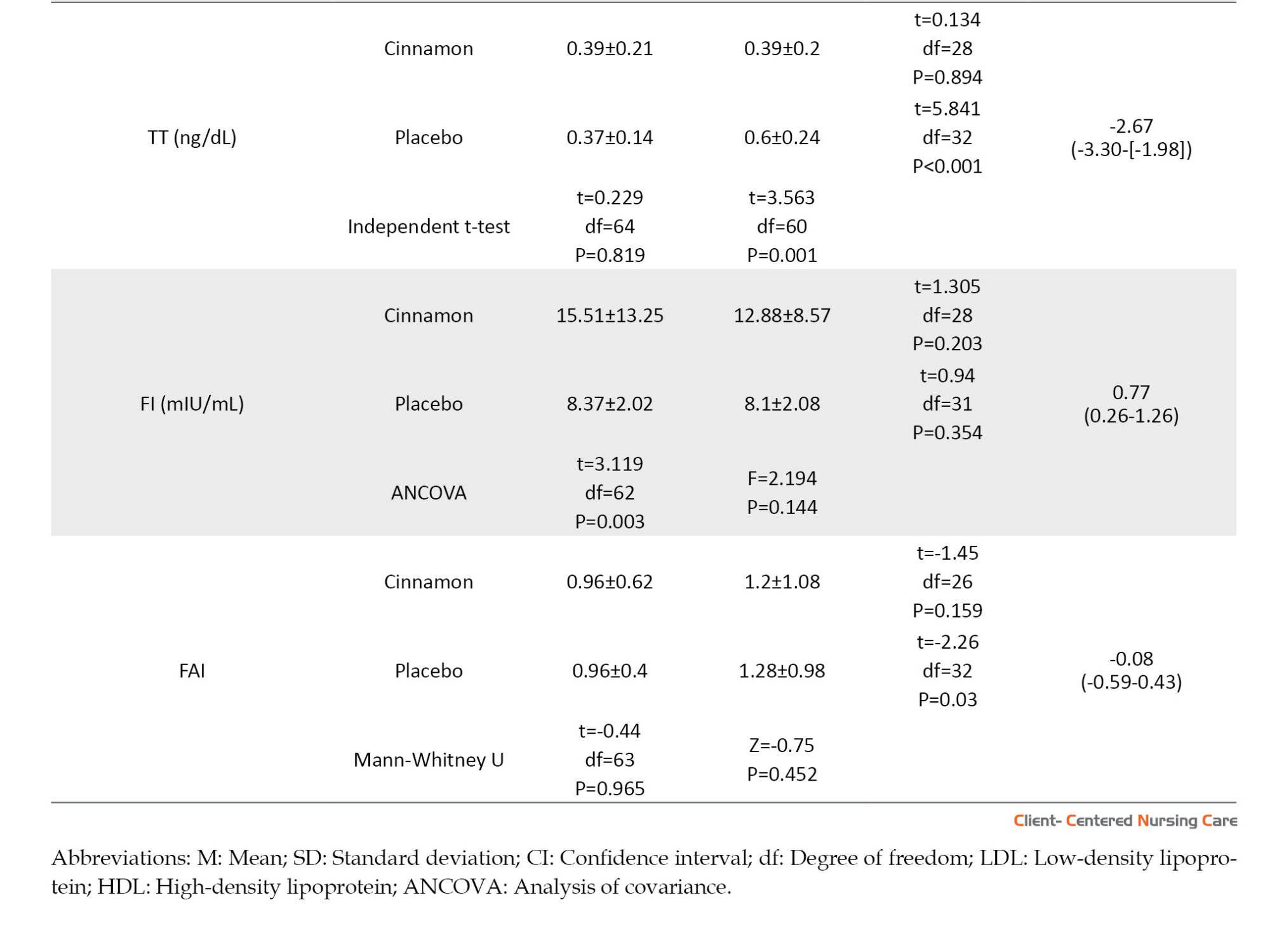
According to Table 3, there was no significant difference between the two groups in terms of all hormonal and metabolic indices changes, except TT changes. That is, an increase in TT in the placebo group at the end of the study was statistically greater than in the cinnamon group (P=0.001).
Discussion
Nowadays, the prevalence of PCOS is increasing and it is becoming a common cause of endocrine and metabolic disorders in women. Nevertheless, PCOS management and treatment approaches remain a challenge (Joshi et al., 2021). However, the mechanisms underlying the effects of herbal extracts on PCOS are needed for further investigations (Arentz et al., 2014). Recently, medicinal plants and herbal compounds have attracted much attention in the management of PCOS due to their lower side effects and appropriate metabolic and hormonal effects in women with PCOS (Joshi et al., 2021). The current study results indicated that taking oral capsules of cinnamon extract at a dose of 3 g/day for 12 consecutive weeks only has a favorable effect on decreasing TT in PCOS women and had no beneficial effects on other studied parameters including FBS, TG, total cholesterol, LDL-C, HDL-C, FI, SHBG, and FAI. The results of different studies in this field have not been the same. For example, in line with our study, Dou et al. (2018) have demonstrated the favorable effects of cinnamon on the hormonal status of the PCOS mouse model (Dou et al., 2018). Blevins et al. (2007) found that cinnamon taken at a dose of 1 g/day for 3 months has no significant effect on fasting glucose, lipid, or insulin levels in subjects with non-insulin-dependent type 2 diabetes (Blevins et al., 2007b). Contrary to our results, the findings of other studies confirmed that cinnamon complementation can significantly reduce FBS, insulin or insulin resistance and raise insulin sensitivity in women with PCOS (Heydarpour et al., 2020; Wahyuningtyas & Sa’adi, 2021). Differences in the metabolic status of subjects may be involved in different results of various outcomes related to glycemic parameters (Borzoei et al., 2018). While our study results did not show any effect of cinnamon on metabolic indices, other studies confirm the positive effects of cinnamon on lipid profile in women with PCOS (Borzoei et al., 2018; Maleki et al., 2021). Since the results concerning the potential effects of cinnamon on metabolic and hormonal profiles are inconsistent, further studies are needed to explore the precise mechanisms of clinical, metabolic, and hormonal changes following cinnamon supplementation, as well as the appropriate dosage in PCOS (Maleki et al., 2021). It is difficult to explain how cinnamon extract could induce its beneficial effects on TT or other hormonal and metabolic indices at the molecular level in women with PCOS. This may be because peroxisome proliferator-activated receptor gamma/alpha, as well as lipid hemostasis target genes, such as lipoprotein lipase (LPL) and CD36 expression, may be induced by cinnamon. In addition, cinnamon with activating AMP-activated protein kinase can suppress acetyl CoA-carboxylase, thereby increasing beta-oxidation and decreasing fatty acid biosynthesis (Maleki et al., 2021). Also, some researchers have suggested that since insulin resistance is the major cause of androgen excess in PCOS women, cinnamon can regulate androgen production by controlling insulin resistance. Cinnamon can downregulate androgen production in PCOS women by reducing advanced glycation end products (Garg & Merhi, 2016; Talaei et al. 2017). In other words, in women with PCOS, hyperinsulinemia can cause ovarian hyperandrogenemia, and it seems that cinnamon can modify the androgen excess status in PCOS women by improving insulin sensitivity (Maleki et al., 2021). This effect is achieved through the insulin receptor autophosphorylation simulation and protein tyrosine phosphatase inhibition by cinnamon. Insulin exerts its effects on androgen production through insulin-like growth factor 1 receptor located on the ovarian theca and stroma cells. Insulin-like growth factor 1, along with insulin-like growth factor binding protein-1 affect the maturation of follicles through paracrine and/or autocrine mechanisms. In this context, cinnamon acts as an amplifier of insulin-receptor function and can also regulate the signaling pathways of insulin and insulin-like growth factor 1 (Dou et al., 2018; Talaei et al. 2017). Nonetheless, in our study, the effect of cinnamon on FI and FBS was not observed. Talaei et al. (2017), who also reported that 3 g of cinnamon per day for eight weeks had no beneficial effect on glycemic indices, such as fasting plasma glucose, suggested that the relative shortening of the intervention period may have contributed to these results. On the other hand, the conflicting results of the effects of cinnamon on metabolic or hormonal status may be relevant to different research communities. For instance, the study by Blevins et al. (2007), which showed the beneficial effects of cinnamon on glycemic markers, has been done on individuals with type 2 diabetes; while the present study on non-diabetic women does not approve these results. The heterogeneity of study reports on cinnamon’s effect on metabolic or hormonal status can be justified by differences in related factors such as duration, dosage, pharmaceutical form of cinnamon use, ethnicity, sample size, and BMI of the study population. Therefore, it is still early to suggest evidence-based cinnamon supplementation and for knowing cinnamon’s mechanism of action and its effects on the human body, large-scale and well-designed clinical trials are needed (Blevins et al., 2007).
Conclusion
In conclusion, we found that the use of oral capsules of cinnamon extract, which is equivalent to 3 grams of cinnamon for 12 consecutive weeks, causes a statistically significant reduction in total TT and has no effect on changes in SHBG, FAI, and metabolic and glycemic parameters in women with PCOS. To better understand the molecular pathways and evaluate the effects of cinnamon in these women, more detailed studies with larger sample sizes are still needed. Besides, it seems future studies should focus on the effects of cinnamon in PCOS women based on their phenotypes.
Study limitations
The findings of this study should be considered in light of certain limitations. An important limitation was that the women included in our study with no PCOS phenotypes. While the PCOS phenotypes may experience different degrees of insulin resistance, the effects of cinnamon on these phenotypes may be different. The other limitation was our participant’s BMI. We studied cinnamon effects on hormonal and metabolic markers of women with PCOS, regardless of their BMI. Therefore, we strongly recommend that more extensive research should be done to determine the effect of cinnamon in women with PCOS according to the weight of women.
Ethical Considerations
Compliance with ethical guidelines
The research protocol was approved by the Ethics Committee of Iran University of Medical Sciences (Code: IR.IUMS.REC.1395.9311373027). Also, the trial protocol was registered in the Iranian Registry of Clinical Trials (IRCT) (Code: IRCT2016021326161N2, date: 31/5/2016). It was performed following the ethical standards of the Declaration of Helsinki (2013 version) and its later amendments or comparable standards of ethics. All participants were initially informed completely about the study goals and methods and then signed an informed written consent form.
Funding
This study was funded and supported by Iran University of Medical Sciences (Grant No.: 95-02-28-28711).
Authors' contributions
Conceptualization: Leila Amini and Sanam Hadipoor; Supervision: Leila Amini; Methodology: Leila Amini and Homa Sadeghi Avval Shahr; Investigation: Sanam Hadipoor, Leila Amini and Afsaneh Ghasemi; Writing the original draft: Sanam Hadipoor, Leila Amini and Bahare Afshar; Review & editing: Leila Amini, Bahare Afshar and Homa Sadeghi Avval Shahr; Data collection: Sanam Hadipoor and Afsaneh Ghasemi; Data analysis: Hamid Haghani; Funding Administration: Leila Amini.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
The authors would like to express their gratitude to the women who participated in this study, as well as Iran University of Medical Sciences for its support and funding.
References
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age. The prevalence of PCOS, according to Rotterdam criteria, is about 6% to 18% (Blagojevic et al., 2017). The prevalence of this disease in various studies conducted in Iran has been reported from 7% to 19.4% (Farhadi-Azar et al., 2022; Ghiasi, 2019). PCOS has received a lot of attention due to its high prevalence and possible consequences such as infertility, and also metabolic and cardiovascular disorders in women. The exact cause of PCOS is unknown; however, it has currently been confirmed that its occurrence is due to genetic and environmental factors (Boyle & Teede, 2012). In addition, some environmental factors, such as diet, physical activity, smoking and stress can also be involved in causing this syndrome (Michalak et al., 2015). Although compensatory insulin resistance and hyperinsulinemia are not diagnostic criteria for PCOS but its prevalence among women with this syndrome is 50%-70% and may reach 95% in overweight women (Qin et al., 2010). PCOS is also the most common cause of infertility, such that 50%-70% of women with infertility due to anovulation have this syndrome (Nasir Amiri et al., 2013). These have made PCOS a frustrating experience for women and a complex scientific challenge for researchers and physicians (Johnson, 2014).
To reduce and treat the physical complications of PCOS and promote the health of women with this syndrome, many pharmacological and non-pharmacological methods have been considered and studied. Complementary therapies in these women have increased over the past 10 years (Smith et al., 2013). One of the common types of alternative drugs is herbal medicine such as saliva officinalis (Amini et al., 2020), curcumin, bebeerine (Joshi et al., 2021), and cinnamon (Baker et al., 2008). Herbal medicines have fewer side effects than conventional treatments (Nagarathna et al., 2014). Cinnamon is a low-risk medicinal plant used daily without any side effects (Rao & Gan, 2014). The use of cinnamon as a potentially useful drug for the treatment of type 2 diabetes began almost about 20 years ago (Sangal, 2011). It has been shown during laboratory and animal studies that cinnamon stimulates insulin secretion (Broadhurst et al., 2000) and cinnamon extract increases the activity of insulin kinase receptors by increasing the activity of phosphatidylinositol 3-kinases (PI3K) in the insulin signaling pathway in the cell, resulting in improved insulin function and increased blood glucose uptake (Heibashy et al., 2013). A study showed the ability of cinnamon to reduce lipid levels in fructose-fed mice (El-Bidawy et al., 2014). The effect of these compounds is also positive in reducing TGs, cholesterol, and low-density lipoprotein (Modaresi et al., 2009). However, another study showed that daily intake of 1 g of cinnamon for three months did not cause significant changes in fasting glucose and glycosylated hemoglobin (Blevins et al., 2007a). Despite several studies that have examined the effects of cinnamon on PCOS aspects, there are controversies in this regard. Accordingly, this study determines the effect of oral cinnamon on metabolic profile in women with PCOS.
Materials and Methods
Study design, setting, and sample
This study was a triple-blinded randomized placebo-controlled clinical trial with parallel groups and was performed on 66 women with PCOS referred to the gynecology and Infertility Clinic of Firoozgar Hospital affiliated with Iran University of Medical Sciences and some selected private centers in Tehran City, Iran, from June 2016 to November 2017 to investigate cinnamon effects on the metabolic status of women with PCOS.
The sample size was calculated based on fasting blood sugar (FBS) mean differences between cinnamon and placebo groups using the Equation 1. It was assumed that a total sample size of 66 subjects (33 participants in each group), would provide an 80% power with a type I error of 0.05 to detect a statistically significant difference in FBS mean between the two studied groups.
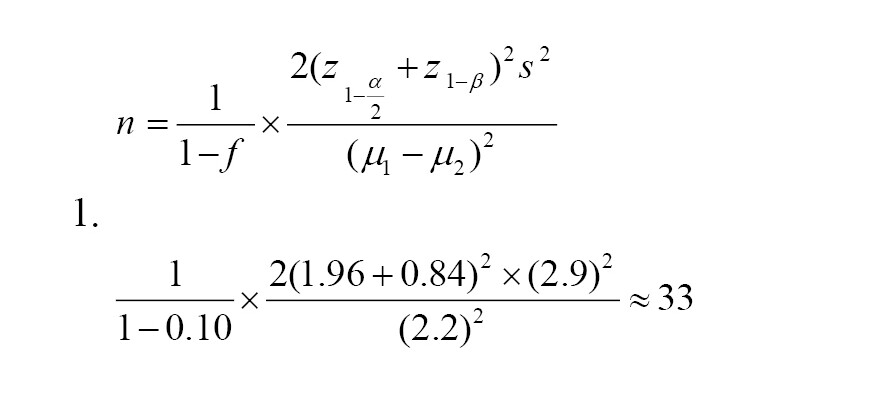
Iranian women aged 18-40 years, with a definitive diagnosis of PCOS by an expert gynecologist, were included in the study according to Rotterdam criteria. The Rotterdam criteria require the presence of two of the following symptoms in the patient: a) Oligo-ovulation or anovulation; b) Hyperandrogenism clinical (such as hirsutism) or biological (including increased free androgen or free testosterone) signs; and c) Polycystic ovaries in ultrasound view (Wang & Mol, 2017). Hirsutism defined as a Ferriman-Gallwey score more than 8 (Ferriman & Gallwey, 1961). In this study, all the women who have used other herbal remedies, glucose or lipid metabolism-affecting drugs, vegetarians, women with body mass index under 19 or over 35 kg/m2, patients with metabolic disorders, and pregnant or nursery women were excluded. Finally, out of 113 participants assessed, 81 women were eligible for the trial and signed an informed consent form. The participants were randomly allocated to cinnamon (n=41) or placebo (n=40) groups by using a randomization list. Randomization was performed by a person who was not involved in the research process. The main outcome measure was metabolic status including FBS, triglyceride (TG), low density cholesterol (LDL and LDL-C), high density cholesterol (HDL and HDL-C), total cholesterol, sex hormone binding globulin (SHBG), total testosterone (TT), fasting insulin (FI), and free androgen index (FAI).
Study measurements
All the subjects completed a demographic and fertility questionnaire, which included information such as age, marital status, duration of education, occupational status, economic status, age of menarche, and body mass index (BMI) using the self-report method before the intervention. Blood test results were recorded before and 12 weeks after intervention by the researcher in a data sheet based on laboratory test reports. All blood samples were collected after 12-14 h of overnight fasting. After taking blood, the samples were placed at room temperature for 20-30 min and then were centrifuged at 2500-3500 revolutions per minute (rpm) for 10-15 min. Blood sera were isolated and stored at -80 ºC for later assessments. All the samples were analyzed in the laboratory of the research in the Laboratory of Gynecology and Infertility Clinic of Firoozgar Hospital. FBS was measured in milligrams per deciliter (mg/dL), TG (mg/dL), and total cholesterol (mg/dL) levels were determined by Pars Azmoon Company enzymatic kits (Tehran, Iran) and Hitachi 912 auto-analyzer (Japan), and levels of LDL-C (mg/dl) and HDL-C (mg/dL) were determined by Pishtaz Teb Company kit (Tehran, Iran) and Hitachi 912 auto-analyzer (Japan). Also, the FI (milli-international units per liter [mIU/L]) was measured by enzyme-linked immunosorbent assay method using the commercial kit (Insulin: Monobind Inc., Lake Forest, CA, USA) with an automated microplate reader (Hyperion Inc., USA). FAI was calculated to determine the androgen status of the subjects using the Equation 2:
2. FAI=(100×total TT [nanomoles per liter or nmol/l])/SHBG (nmol/L)
Also, BMI was calculated using the Equation 3:
3. BMI (kg/m2)=weight (kg)/ height2 [m]
Variables were measured twice, once before the intervention and once 12 weeks after the onset of the intervention.
Preparations of the cinnamon extract capsules and the placebo
The cinnamon was taken from an herbal market in Tehran City, Iran, and was approved by a pharmacognosy expert at the School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The appropriate dosage was selected based on the recommended dosage in Iranian Traditional Medicine and physician’s desk reference for herbal medicines. According to the mentioned references, the average dose of 3 g/day was selected. In the next step, cinnamon was ground using an electrical grinder, and the resulting powder was combined with 96º ethanol as the solvent. After the solvent evaporation, the remaining extract was mixed with corn starch and turned into a powder. A total of 3 g of that powder then were placed into each size 0 capsule manually. For the placebo, the same capsules were filled with corn starch alone. Finally, the capsules were packaged and coded in similar boxes and named A or B by a person independent of the research team. All of the participants, researchers, and statisticians were blinded to the groups.
After initial measurements, intervention was started. Some of the subjects in both groups withdrew during the follow-up (Figure 1). Participants in the cinnamon group (n=33) took the cinnamon extract capsules 3 g/day orally for twelve consecutive weeks, and the placebo group (n=33) simultaneously and in parallel took placebo capsules orally once a day for twelve consecutive weeks. During the study period, the subjects were monitored by telephone once or twice a week and were asked to report any drug side effects, or complications, or stop taking the drug. Women who did not take more than 2 capsules for any reason, were excluded from the study (Figure 1).

Statistical analysis
The normal distribution of the data was checked by the Kolmogorov-Smirnov test and graphical methods. Data was presented as Mean±SD for quantitative data or frequency and percent (for qualitative data) in tables. The Student t-test was used for between-group comparison of quantitative variables with normal distribution, while the paired t-test was used to compare them before and after the intervention in each group. The Mann-Whitney U test was used for between-group comparison of quantitative variables without a normal distribution. Categorical variables were compared using the chi-square test. Analysis of covariance was used when there were significant differences between the groups before the intervention. All statistical analyses were performed using the SPSS software, version 16 (SPSS Inc., Chicago, IL, USA). Meanwhile, the significance level was set at P<0.05.
Results
The mean age of the subjects was 27.4±78.9 and 26.31±4.0 in the intervention and control groups, respectively. The characteristics of the subjects are summarized in Table 1. Accordingly, the random allocation could balance between the two groups in terms of the demographic and reproductive characteristics of the participants. Therefore, there were no statistically significant differences between the groups regarding these variables.

The results of the independent t-test in Table 2 show that there are no significant differences between the groups before intervention regarding FBS, TG, LDL-C, HDL-C, SHBG, TT, and FAI; however, total cholesterol (P=0.015) and FI (P=0.003) were statistically lower in the placebo group than the cinnamon group. At the end of the study, the two groups did not show any significant differences in terms of FBS, TG, LDL-C, HDL-C, total cholesterol, SHBG, and FAI; however, the women in the cinnamon group had a significantly lower TT than other group (P=0.001). Also, the results of the paired t-test showed that in the placebo group, FBS, LDL-C, HDL-C, total cholesterol, TT, SHBG, and FAI had a significant increase at the end of the study (P<0.05); however, TG and FI did not show any statistical changes. In the cinnamon group, only HDL-C showed a significant increase after the intervention compared to before (P=0.042), and the other hormones and metabolic indicators remained unchanged. Since total cholesterol and FI were significantly lower in the placebo group compared to the cinnamon group, the analysis of covariance was used to compare the mentioned variables between the two groups at the end of the study. The results showed that there were no significant differences between the two groups regarding total cholesterol and FI (Table 2).


According to Table 3, there was no significant difference between the two groups in terms of all hormonal and metabolic indices changes, except TT changes. That is, an increase in TT in the placebo group at the end of the study was statistically greater than in the cinnamon group (P=0.001).

Discussion
Nowadays, the prevalence of PCOS is increasing and it is becoming a common cause of endocrine and metabolic disorders in women. Nevertheless, PCOS management and treatment approaches remain a challenge (Joshi et al., 2021). However, the mechanisms underlying the effects of herbal extracts on PCOS are needed for further investigations (Arentz et al., 2014). Recently, medicinal plants and herbal compounds have attracted much attention in the management of PCOS due to their lower side effects and appropriate metabolic and hormonal effects in women with PCOS (Joshi et al., 2021). The current study results indicated that taking oral capsules of cinnamon extract at a dose of 3 g/day for 12 consecutive weeks only has a favorable effect on decreasing TT in PCOS women and had no beneficial effects on other studied parameters including FBS, TG, total cholesterol, LDL-C, HDL-C, FI, SHBG, and FAI. The results of different studies in this field have not been the same. For example, in line with our study, Dou et al. (2018) have demonstrated the favorable effects of cinnamon on the hormonal status of the PCOS mouse model (Dou et al., 2018). Blevins et al. (2007) found that cinnamon taken at a dose of 1 g/day for 3 months has no significant effect on fasting glucose, lipid, or insulin levels in subjects with non-insulin-dependent type 2 diabetes (Blevins et al., 2007b). Contrary to our results, the findings of other studies confirmed that cinnamon complementation can significantly reduce FBS, insulin or insulin resistance and raise insulin sensitivity in women with PCOS (Heydarpour et al., 2020; Wahyuningtyas & Sa’adi, 2021). Differences in the metabolic status of subjects may be involved in different results of various outcomes related to glycemic parameters (Borzoei et al., 2018). While our study results did not show any effect of cinnamon on metabolic indices, other studies confirm the positive effects of cinnamon on lipid profile in women with PCOS (Borzoei et al., 2018; Maleki et al., 2021). Since the results concerning the potential effects of cinnamon on metabolic and hormonal profiles are inconsistent, further studies are needed to explore the precise mechanisms of clinical, metabolic, and hormonal changes following cinnamon supplementation, as well as the appropriate dosage in PCOS (Maleki et al., 2021). It is difficult to explain how cinnamon extract could induce its beneficial effects on TT or other hormonal and metabolic indices at the molecular level in women with PCOS. This may be because peroxisome proliferator-activated receptor gamma/alpha, as well as lipid hemostasis target genes, such as lipoprotein lipase (LPL) and CD36 expression, may be induced by cinnamon. In addition, cinnamon with activating AMP-activated protein kinase can suppress acetyl CoA-carboxylase, thereby increasing beta-oxidation and decreasing fatty acid biosynthesis (Maleki et al., 2021). Also, some researchers have suggested that since insulin resistance is the major cause of androgen excess in PCOS women, cinnamon can regulate androgen production by controlling insulin resistance. Cinnamon can downregulate androgen production in PCOS women by reducing advanced glycation end products (Garg & Merhi, 2016; Talaei et al. 2017). In other words, in women with PCOS, hyperinsulinemia can cause ovarian hyperandrogenemia, and it seems that cinnamon can modify the androgen excess status in PCOS women by improving insulin sensitivity (Maleki et al., 2021). This effect is achieved through the insulin receptor autophosphorylation simulation and protein tyrosine phosphatase inhibition by cinnamon. Insulin exerts its effects on androgen production through insulin-like growth factor 1 receptor located on the ovarian theca and stroma cells. Insulin-like growth factor 1, along with insulin-like growth factor binding protein-1 affect the maturation of follicles through paracrine and/or autocrine mechanisms. In this context, cinnamon acts as an amplifier of insulin-receptor function and can also regulate the signaling pathways of insulin and insulin-like growth factor 1 (Dou et al., 2018; Talaei et al. 2017). Nonetheless, in our study, the effect of cinnamon on FI and FBS was not observed. Talaei et al. (2017), who also reported that 3 g of cinnamon per day for eight weeks had no beneficial effect on glycemic indices, such as fasting plasma glucose, suggested that the relative shortening of the intervention period may have contributed to these results. On the other hand, the conflicting results of the effects of cinnamon on metabolic or hormonal status may be relevant to different research communities. For instance, the study by Blevins et al. (2007), which showed the beneficial effects of cinnamon on glycemic markers, has been done on individuals with type 2 diabetes; while the present study on non-diabetic women does not approve these results. The heterogeneity of study reports on cinnamon’s effect on metabolic or hormonal status can be justified by differences in related factors such as duration, dosage, pharmaceutical form of cinnamon use, ethnicity, sample size, and BMI of the study population. Therefore, it is still early to suggest evidence-based cinnamon supplementation and for knowing cinnamon’s mechanism of action and its effects on the human body, large-scale and well-designed clinical trials are needed (Blevins et al., 2007).
Conclusion
In conclusion, we found that the use of oral capsules of cinnamon extract, which is equivalent to 3 grams of cinnamon for 12 consecutive weeks, causes a statistically significant reduction in total TT and has no effect on changes in SHBG, FAI, and metabolic and glycemic parameters in women with PCOS. To better understand the molecular pathways and evaluate the effects of cinnamon in these women, more detailed studies with larger sample sizes are still needed. Besides, it seems future studies should focus on the effects of cinnamon in PCOS women based on their phenotypes.
Study limitations
The findings of this study should be considered in light of certain limitations. An important limitation was that the women included in our study with no PCOS phenotypes. While the PCOS phenotypes may experience different degrees of insulin resistance, the effects of cinnamon on these phenotypes may be different. The other limitation was our participant’s BMI. We studied cinnamon effects on hormonal and metabolic markers of women with PCOS, regardless of their BMI. Therefore, we strongly recommend that more extensive research should be done to determine the effect of cinnamon in women with PCOS according to the weight of women.
Ethical Considerations
Compliance with ethical guidelines
The research protocol was approved by the Ethics Committee of Iran University of Medical Sciences (Code: IR.IUMS.REC.1395.9311373027). Also, the trial protocol was registered in the Iranian Registry of Clinical Trials (IRCT) (Code: IRCT2016021326161N2, date: 31/5/2016). It was performed following the ethical standards of the Declaration of Helsinki (2013 version) and its later amendments or comparable standards of ethics. All participants were initially informed completely about the study goals and methods and then signed an informed written consent form.
Funding
This study was funded and supported by Iran University of Medical Sciences (Grant No.: 95-02-28-28711).
Authors' contributions
Conceptualization: Leila Amini and Sanam Hadipoor; Supervision: Leila Amini; Methodology: Leila Amini and Homa Sadeghi Avval Shahr; Investigation: Sanam Hadipoor, Leila Amini and Afsaneh Ghasemi; Writing the original draft: Sanam Hadipoor, Leila Amini and Bahare Afshar; Review & editing: Leila Amini, Bahare Afshar and Homa Sadeghi Avval Shahr; Data collection: Sanam Hadipoor and Afsaneh Ghasemi; Data analysis: Hamid Haghani; Funding Administration: Leila Amini.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
The authors would like to express their gratitude to the women who participated in this study, as well as Iran University of Medical Sciences for its support and funding.
References
Amini, L., et al., 2020. Efficacy of Salvia officinalis extract on the prevention of insulin resistance in euglycemic patients with polycystic ovary syndrome: A double-blinded placebo-controlled clinical trial. Complementary Therapies in Medicine, 48, pp. 102245. [DOI:10.1016/j.ctim.2019.102245] [PMID]
Amiri, F. N., Simbar, M. & Tahamtan, R. A. M., 2013. [Components of quality of life of women with polycystic ovary syndrome: A qualitative study (Persian)]. Payesh (Health Monitor), 12(6), pp. 657-69. [Link]
Arentz, S., et al., 2014. Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. BMC Complementary and Alternative Medicine, 14, pp. 511. [DOI:10.1186/1472-6882-14-511] [PMID]
Baker, W. L., et al., 2008. Effect of cinnamon on glucose control and lipid parameters. Diabetes Care, 31(1), pp. 41–3. [DOI:10.2337/dc07-1711] [PMID]
Blagojevic, I. P., et al., 2017. Women with polycystic ovary syndrome and risk of cardiovascular disease. Journal of Medical Biochemistry, 36(3), pp. 259–69. [DOI:10.1515/jomb-2017-0020] [PMID]
Blevins, S. M., 2007. Effect of cinnamon on glucose and lipid levels in non-insulin-dependent type 2 diabetes. Diabetes Care, 30(9), pp. 2236–7. [DOI:10.2337/dc07-0098] [PMID]
Blevins, S. M., et al., 2007. Effect of cinnamon on glucose and lipid levels in non insulin-dependent type 2 diabetes. Diabetes Care, 30(9), pp. 2236-7. [DOI:10.2337/dc07-0098] [PMID]
Borzoei, A., Rafraf, M. & Asghari-Jafarabadi, M., 2018. Cinnamon improves metabolic factors without detectable effects on adiponectin in women with polycystic ovary syndrome. Asia Pacific Journal of Clinical Nutrition, 27(3), pp. 556–63. [PMID]
Boyle, J. & Teede, H. J., 2012. Polycystic ovary syndrome: An update. Australian Family Physician, 41(10), pp. 752–6. [PMID]
Broadhurst, C. L., Polansky, M. M. & Anderson, R. A., 2000. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. Journal of Agricultural and Food Chemistry, 48(3), pp. 849–52. [DOI:10.1021/jf9904517] [PMID]
Dou, L., 2018. The effect of cinnamon on polycystic ovary syndrome in a mouse model. Reproductive Biology and Endocrinology: RB&E, 16(1), pp. 99. [DOI:10.1186/s12958-018-0418-y] [PMID]
EL-Bidawy, M. H., et al., 2014. Effects of fat and cinnamon feeding on the diabetogenic state after rat injection with dexamethasone. Life Science Journal, 11(1), pp. 282-8. [Link]
Farhadi-Azar, M., et al., 2022. The prevalence of polycystic ovary syndrome, its phenotypes and cardio-metabolic features in a community sample of Iranian Population: Tehran lipid and glucose study. Frontiers in Endocrinology, 13, pp. 825528.[DOI:10.3389/fendo.2022.825528] [PMID]
Ferriman, D. & Gallwey, J., 1961. Clinical assessment of body hair growth in women. The Journal of Clinical Endocrinology & Metabolism, 21, pp. 1440-7. [DOI:10.1210/jcem-21-11-1440] [PMID]
Ghiasi, A., 2019. Prevalence of polycystic ovarian syndrome in Iranian adolescents: A systematic review and meta-analysis. Journal of South Asian Federation of Obstetrics and Gynaecology, 11(3), pp. 194-7. [DOI:10.5005/jp-journals-10006-1681]
Garg, D. & Merhi, Z., 2016. Relationship between advanced glycation end products and steroidogenesis in PCOS. Reproductive Biology and Endocrinology : RB&E, 14(1), pp. 71. [DOI:10.1186/s12958-016-0205-6] [PMID]
Heibashy, M. I. A., Mazen, G. M. A. & SHahin, M. I., 2013. Metabolic changes and hormonal disturbances in polycystic ovarian syndrome rats and the amelioration effects of metformin and/or cinnamon extraction. The Journal of American Science, 9(12), pp. 54-62. [Link]
Heydarpour, F., et al., 2020. Effects of cinnamon on controlling metabolic parameters of polycystic ovary syndrome: A systematic review and meta-analysis. Journal of Ethnopharmacology, 254, pp. 112741. [DOI:10.1016/j.jep.2020.112741] [PMID]
Johnson N. P., 2014. Metformin use in women with polycystic ovary syndrome. Annals of Translational Medicine, 2(6), pp. 56. [DOI:10.3978/j.issn.2305-5839.2014.04.15] [PMID]
Joshi, M., et al., 2021. Polycystic Ovarian syndrome: A review covering phytoconstituents for its outstrip management. Pharmacological Research - Modern Chinese Medicine, 1, pp. 100011. [DOI:10.1016/j.prmcm.2021.100011]
Maleki, V., et al., 2021. Mechanistic and therapeutic insight into the effects of cinnamon in polycystic ovary syndrome: A systematic review. Journal of Ovarian Research, 14(1), pp. 130. [DOI:10.1186/s13048-021-00870-5] [PMID]
MIichalak, R. M., Jagodzińska, A. & Zieleniewski, W., 2015. The prevalence of high blood pressure as one of cardiovascular risk factors among women with polycystic ovary syndrome. Arterial Hypertension, 19(1), pp. 19-22. [Link]
Modaresi, M., Messripour, M. & Rajaei, R., 2009. [The effect of cinnamon (bark) extract on male reproductive physiology in mice (Persian)]. Armaghane Danesh, 14(1), pp. 67-77. [Link]
Nagarathna, P., Rajan, P. R. & Koneri, R., 2014. A detailed study on poly cystic ovarian syndrome and it’s treatment with natural products. International Journal of Toxicological and Pharmacological Research, 5(4), pp. 109-20. [Link]
Qin, B., Panickar, K. S. & Anderson, R. A., 2010. Cinnamon: Potential role in the prevention of insulin resistance, metabolic syndrome, and type 2 diabetes. Journal of Diabetes Science and Technology, 4(3), pp. 685–93. [DOI:10.1177/193229681000400324] [PMID]
Rao, P. V. & Gan, S. H., 2014. Cinnamon: A multifaceted medicinal plant. Evidence-based Complementary and Alternative Medicine: eCAM, 2014, pp. 642942. [DOI:10.1155/2014/642942] [PMID]
Sangal, A., 2011. Role of cinnamon as beneficial antidiabetic food adjunct: A review. Advances in Applied Science Research, 2(4), pp. 440-50. [Link]
Smith, C. A., Bateson, D. J. & Weisberg, E., 2013. A survey describing the use of complementary therapies and medicines by women attending a family planning clinic. BMC Complementary and Alternative Medicine, 13, pp. 224. [DOI:10.1186/1472-6882-13-224] [PMID]
Talaei B, et al., 2017. Effects of cinnamon consumption on glycemic indicators, advanced glycation end products, and antioxidant status in type 2 diabetic patients. Nutrients, 9(9), pp. 991. [DOI:10.3390/nu9090991] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2024/04/18 | Accepted: 2024/09/3 | Published: 2025/02/1
Received: 2024/04/18 | Accepted: 2024/09/3 | Published: 2025/02/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



